当前位置:
X-MOL 学术
›
Chem. Phys. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H-bonding behavior of ethylene oxide within the clathrate hydrates revisited: Experiment and theory
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-06-29 , DOI: 10.1016/j.cplett.2020.137728 Zafer Maşlakcı , J. Paul Devlin , Nevin Uras-Aytemiz
中文翻译:

环氧乙烷在包合物水合物中的H键行为:实验与理论
更新日期:2020-07-06
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-06-29 , DOI: 10.1016/j.cplett.2020.137728 Zafer Maşlakcı , J. Paul Devlin , Nevin Uras-Aytemiz
|
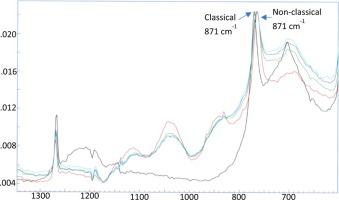
FTIR spectroscopy has been used to reexplore the nonclassical behavior of ethylene oxide (EO) within the large cages of clathrate hydrates. In most of the spectroscopic studies of EO within the clathrate hydrate cages, the classical EO bands attributed to the C-O stretch mode of EO were misassigned. Therefore, the all-vapor sub-second approach to clathrate-hydrate formation combined with computational studies was used to reexamine spectroscopic characteristics of EO molecules in which they can be either in classical or nonclassical forms.
中文翻译:

环氧乙烷在包合物水合物中的H键行为:实验与理论
FTIR光谱已用于探索笼形水合物大笼子中环氧乙烷(EO)的非经典行为。在笼形水合物笼中的大多数EO光谱研究中,归因于EO的CO拉伸模式的经典EO谱带被错误分配。因此,将亚蒸汽包合物水合物形成的全亚秒方法与计算研究相结合,可以重新检查EO分子的光谱特征,其中它们可以是经典形式也可以是非经典形式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号