当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biochemical determinants of the IGFBP-3-hyaluronan interaction.
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-07-22 , DOI: 10.1002/2211-5463.12919 Sadaf Dorandish 1 , Jonathan Devos 1 , Bradley Clegg 1 , Deanna Price 1 , Robert Muterspaugh 1 , Jeffrey Guthrie 1 , Deborah L Heyl 1 , Hedeel Guy Evans 1
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-07-22 , DOI: 10.1002/2211-5463.12919 Sadaf Dorandish 1 , Jonathan Devos 1 , Bradley Clegg 1 , Deanna Price 1 , Robert Muterspaugh 1 , Jeffrey Guthrie 1 , Deborah L Heyl 1 , Hedeel Guy Evans 1
Affiliation
|
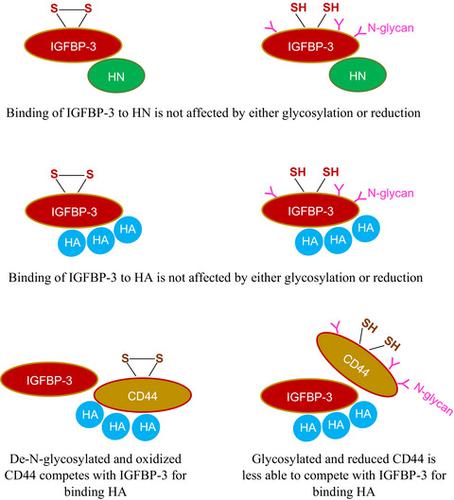
IGFBP‐3, the most abundant IGFBP and the main carrier of insulin‐like growth factor I (IGF‐I) in the circulation, can bind IGF‐1 with high affinity, which attenuates IGF/IGF‐IR interactions, thereby resulting in antiproliferative effects. The C‐terminal domain of insulin‐like growth factor‐binding protein‐3 (IGFBP‐3) is known to contain an 18‐basic amino acid motif capable of interacting with either humanin (HN) or hyaluronan (HA). We previously showed that the 18‐amino acid IGFBP‐3 peptide is capable of binding either HA or HN with comparable affinities to the full‐length IGFBP‐3 protein and that IGFBP‐3 can compete with the HA receptor, CD44, for binding HA. Blocking the interaction between HA and CD44 reduced viability of A549 human lung cancer cells. In this study, we set out to better characterize IGFBP‐3‐HA interactions. We show that both stereochemistry and amino acid identity are important determinants of the interaction between the IGFBP‐3 peptide and HA and for the peptide's ability to exert its cytotoxic effects. Binding of IGFBP‐3 to either HA or HN was unaffected by glycosylation or reduction of IGFBP‐3, suggesting that the basic 18‐amino acid residue sequence of IGFBP‐3 remains accessible for interaction with either HN or HA upon glycosylation or reduction of the full‐length protein. Removing N‐linked oligosaccharides from CD44 increased its ability to compete with IGFBP‐3 for binding HA, while reduction of CD44 rendered the protein relatively ineffective at blocking IGFBP‐3‐HA interactions. We conclude that both deglycosylation and disulfide bond formation are important for CD44 to compete with IGFBP‐3 for binding HA.
中文翻译:

IGFBP-3-透明质酸相互作用的生化决定因素。
IGFBP-3是循环中含量最丰富的IGFBP,也是胰岛素样生长因子I(IGF-I)的主要载体,可以高亲和力结合IGF-1,减弱IGF/IGF-IR相互作用,从而产生抗增殖作用。影响。已知胰岛素样生长因子结合蛋白 3 (IGFBP-3) 的 C 端结构域包含一个 18 个碱性氨基酸基序,能够与护脑素 (HN) 或透明质酸 (HA) 相互作用。我们之前表明,18 个氨基酸的 IGFBP-3 肽能够结合 HA 或 HN,与全长 IGFBP-3 蛋白具有相当的亲和力,并且 IGFBP-3 可以与 HA 受体 CD44 竞争结合 HA 。阻断 HA 和 CD44 之间的相互作用会降低 A549 人肺癌细胞的活力。在本研究中,我们着手更好地表征 IGFBP-3-HA 相互作用。我们表明,立体化学和氨基酸同一性都是 IGFBP-3 肽与 HA 之间相互作用以及肽发挥细胞毒性作用能力的重要决定因素。 IGFBP-3 与 HA 或 HN 的结合不受 IGFBP-3 糖基化或还原的影响,表明 IGFBP-3 的基本 18 个氨基酸残基序列在糖基化或还原后仍可与 HN 或 HA 相互作用。全长蛋白质。从 CD44 中去除 N 连接寡糖增加了其与 IGFBP-3 竞争结合 HA 的能力,而 CD44 的减少则使该蛋白质在阻断 IGFBP-3-HA 相互作用方面相对无效。我们的结论是,去糖基化和二硫键形成对于 CD44 与 IGFBP-3 竞争结合 HA 都很重要。
更新日期:2020-07-22
中文翻译:

IGFBP-3-透明质酸相互作用的生化决定因素。
IGFBP-3是循环中含量最丰富的IGFBP,也是胰岛素样生长因子I(IGF-I)的主要载体,可以高亲和力结合IGF-1,减弱IGF/IGF-IR相互作用,从而产生抗增殖作用。影响。已知胰岛素样生长因子结合蛋白 3 (IGFBP-3) 的 C 端结构域包含一个 18 个碱性氨基酸基序,能够与护脑素 (HN) 或透明质酸 (HA) 相互作用。我们之前表明,18 个氨基酸的 IGFBP-3 肽能够结合 HA 或 HN,与全长 IGFBP-3 蛋白具有相当的亲和力,并且 IGFBP-3 可以与 HA 受体 CD44 竞争结合 HA 。阻断 HA 和 CD44 之间的相互作用会降低 A549 人肺癌细胞的活力。在本研究中,我们着手更好地表征 IGFBP-3-HA 相互作用。我们表明,立体化学和氨基酸同一性都是 IGFBP-3 肽与 HA 之间相互作用以及肽发挥细胞毒性作用能力的重要决定因素。 IGFBP-3 与 HA 或 HN 的结合不受 IGFBP-3 糖基化或还原的影响,表明 IGFBP-3 的基本 18 个氨基酸残基序列在糖基化或还原后仍可与 HN 或 HA 相互作用。全长蛋白质。从 CD44 中去除 N 连接寡糖增加了其与 IGFBP-3 竞争结合 HA 的能力,而 CD44 的减少则使该蛋白质在阻断 IGFBP-3-HA 相互作用方面相对无效。我们的结论是,去糖基化和二硫键形成对于 CD44 与 IGFBP-3 竞争结合 HA 都很重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号