Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A study on the structure, mechanism, and biochemistry of kanamycin B dioxygenase (KanJ)—an enzyme with a broad range of substrates
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-06-27 , DOI: 10.1111/febs.15462 Beata Mrugała 1 , Anna Miłaczewska 1 , Przemyslaw Jerzy Porebski 1, 2 , Ewa Niedzialkowska 1, 2 , Maciej Guzik 1 , Wladek Minor 2 , Tomasz Borowski 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-06-27 , DOI: 10.1111/febs.15462 Beata Mrugała 1 , Anna Miłaczewska 1 , Przemyslaw Jerzy Porebski 1, 2 , Ewa Niedzialkowska 1, 2 , Maciej Guzik 1 , Wladek Minor 2 , Tomasz Borowski 1
Affiliation
|
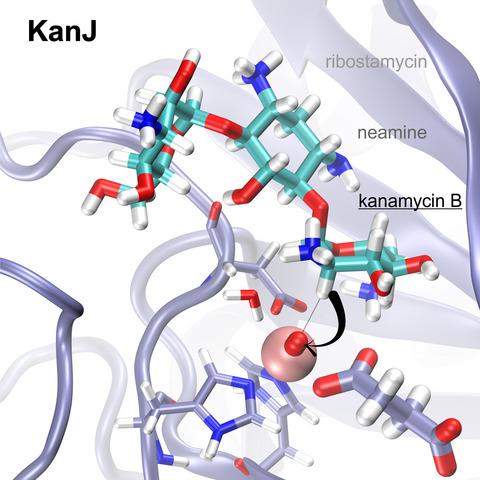
Kanamycin A is an aminoglycoside antibiotic isolated from Streptomyces kanamyceticus and used against a wide spectrum of bacteria, including Mycobacterium tuberculosis. Biosynthesis of kanamycin involves an oxidative deamination step catalyzed by kanamycin B dioxygenase (KanJ), thereby the C2’ position of kanamycin B is transformed into a keto group upon release of ammonia. Here, we present for the first time, structural models of KanJ with several ligands, which along with the results of ITC binding assays and HPLC activity tests explain substrate specificity of the enzyme. The large size of the binding pocket suggests that KanJ can accept a broad range of substrates, which was confirmed by activity tests. Specificity of the enzyme with respect to its substrate is determined by the hydrogen bond interactions between the methylamino group of the antibiotic and highly conserved Asp134 and Cys150 as well as between hydroxyl groups of the substrate and Asn120 and Gln80. Upon antibiotic binding, the C terminus loop is significantly rearranged and Gln80 and Asn120, which are directly involved in substrate recognition, change their conformations. Based on reaction energy profiles obtained by density functional theory (DFT) simulations, we propose a mechanism of ketone formation involving the reactive FeIV = O and proceeding either via OH rebound, which yields a hemiaminal intermediate or by abstraction of two hydrogen atoms, which leads to an imine species. At acidic pH, the latter involves a lower barrier than the OH rebound, whereas at basic pH, the barrier leading to an imine vanishes completely.
中文翻译:

卡那霉素 B 双加氧酶 (KanJ) 的结构、机制和生物化学研究——一种具有广泛底物的酶
卡那霉素 A 是一种从卡那霉素链霉菌中分离出来的氨基糖苷类抗生素,用于对抗多种细菌,包括结核分枝杆菌. 卡那霉素的生物合成涉及由卡那霉素 B 双加氧酶 (KanJ) 催化的氧化脱氨步骤,因此卡那霉素 B 的 C2' 位置在释放氨时转化为酮基。在这里,我们首次展示了具有几种配体的 KanJ 结构模型,连同 ITC 结合测定和 HPLC 活性测试的结果解释了酶的底物特异性。结合口袋的大尺寸表明 KanJ 可以接受广泛的底物,这已通过活性测试得到证实。酶对其底物的特异性由抗生素的甲氨基与高度保守的 Asp134 和 Cys150 之间以及底物的羟基与 Asn120 和 Gln80 之间的氢键相互作用决定。与抗生素结合后,C末端环明显重排,直接参与底物识别的Gln80和Asn120改变了它们的构象。基于通过密度泛函理论 (DFT) 模拟获得的反应能量分布,我们提出了一种涉及反应性 Fe 的酮形成机制IV = O 并通过 OH 反弹进行,产生半胺中间体或通过提取两个氢原子,产生亚胺物质。在酸性 pH 下,后者涉及比 OH 反弹更低的屏障,而在碱性 pH 下,导致亚胺的屏障完全消失。
更新日期:2020-06-27
中文翻译:

卡那霉素 B 双加氧酶 (KanJ) 的结构、机制和生物化学研究——一种具有广泛底物的酶
卡那霉素 A 是一种从卡那霉素链霉菌中分离出来的氨基糖苷类抗生素,用于对抗多种细菌,包括结核分枝杆菌. 卡那霉素的生物合成涉及由卡那霉素 B 双加氧酶 (KanJ) 催化的氧化脱氨步骤,因此卡那霉素 B 的 C2' 位置在释放氨时转化为酮基。在这里,我们首次展示了具有几种配体的 KanJ 结构模型,连同 ITC 结合测定和 HPLC 活性测试的结果解释了酶的底物特异性。结合口袋的大尺寸表明 KanJ 可以接受广泛的底物,这已通过活性测试得到证实。酶对其底物的特异性由抗生素的甲氨基与高度保守的 Asp134 和 Cys150 之间以及底物的羟基与 Asn120 和 Gln80 之间的氢键相互作用决定。与抗生素结合后,C末端环明显重排,直接参与底物识别的Gln80和Asn120改变了它们的构象。基于通过密度泛函理论 (DFT) 模拟获得的反应能量分布,我们提出了一种涉及反应性 Fe 的酮形成机制IV = O 并通过 OH 反弹进行,产生半胺中间体或通过提取两个氢原子,产生亚胺物质。在酸性 pH 下,后者涉及比 OH 反弹更低的屏障,而在碱性 pH 下,导致亚胺的屏障完全消失。











































 京公网安备 11010802027423号
京公网安备 11010802027423号