European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-06-27 , DOI: 10.1016/j.ejmech.2020.112519 Gang Li 1 , Yuxi Wang 2 , Ling Li 3 , Yichang Ren 3 , Xin Deng 3 , Jin Liu 3 , Wei Wang 3 , Meihua Luo 1 , Shuwen Liu 3 , Jianjun Chen 3
|
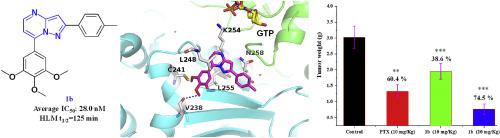
A series of Pyrazolo[1,5-a]Pyrimidine analogs were designed and synthesized as novel tubulin inhibitors. Among them, compounds 1a and 1b showed the highest antiproliferative activity against a panel of cancer cell lines with average IC50 values of 24.8 nM and 28 nM, respectively. We determined the crystal structures of 1a and 1b in complex with tubulin and confirmed their direct binding to the colchicine site. Compounds 1a and 1b also effectively inhibited tubulin polymerization in vitro, induced cell cycle arrest in G2/M phase, and inhibited cancer cell migration. In addition, compound 1b exhibited high metabolic stability in human liver microsomes. Finally, 1b was highly effective in suppressing tumor growth in a B16–F10 mouse melanoma model without apparent toxicity. In summary, these results suggest that 1b represents a promising tubulin inhibitor worthy of further investigation.
中文翻译:

吡唑并[1,5-a]嘧啶衍生物作为微管蛋白聚合抑制剂的设计,合成和生物评价,其靶向具有秋水仙碱结合位点的强抗癌活性。
设计并合成了一系列吡唑并[1,5- a ]嘧啶类似物,作为新型微管蛋白抑制剂。其中,化合物1a和1b对一组癌细胞具有最高的抗增殖活性,平均IC 50值分别为24.8 nM和28 nM。我们确定了与微管蛋白复合的1a和1b的晶体结构,并确认了它们与秋水仙碱位点的直接结合。化合物1a和1b在体外也有效抑制微管蛋白聚合,诱导细胞周期停滞在G2 / M期,并抑制癌细胞迁移。另外,化合物1b在人肝微粒体中表现出高的代谢稳定性。最后,在b16–f10小鼠黑色素瘤模型中,1b在抑制肿瘤生长方面非常有效,没有明显的毒性。总而言之,这些结果表明1b代表有希望的微管蛋白抑制剂,值得进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号