当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A fully synthetic 6-aza-artemisinin bearing an amphiphilic chain generates aggregates and exhibits anti-cancer activities.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-26 , DOI: 10.1039/d0ob00919a Hikari Koi 1 , Norihito Takahashi 1 , Yasufumi Fuchi 2 , Tomohiro Umeno 2 , Yukiko Muramatsu 3 , Hiroyuki Seimiya 3 , Satoru Karasawa 2 , Hiroki Oguri 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-26 , DOI: 10.1039/d0ob00919a Hikari Koi 1 , Norihito Takahashi 1 , Yasufumi Fuchi 2 , Tomohiro Umeno 2 , Yukiko Muramatsu 3 , Hiroyuki Seimiya 3 , Satoru Karasawa 2 , Hiroki Oguri 4
Affiliation
|
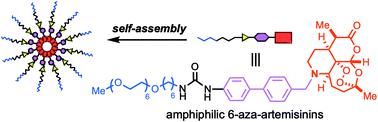
Installation of a nitrogen at the C6 position of artemisinin facilitates the addition of a functional unit on the cyclohexane moiety (C-ring). In this study, conjugation of an amphiphilic chain, composed of sequentially connected hydrophilic oligoethylene glycol, hydrophobic alkyl chain, urea, and 4,4′-disubstituted biphenyl linker, imparted self-assembling properties. The fully synthetic mid-molecular weight 6-aza-artemisinin 6 bearing the amphiphilic moiety formed aggregates (approx. 200 nm) at ambient temperature and exhibited increased in vitro anti-cancer activities compared to the N-benzylated aza-artemisinin 5.
中文翻译:

带有两亲链的完全合成的 6-氮杂青蒿素会产生聚集体并表现出抗癌活性。
在青蒿素的 C6 位置安装一个氮有利于在环己烷部分(C 环)上添加一个功能单元。在这项研究中,由顺序连接的亲水低聚乙二醇、疏水烷基链、尿素和 4,4'-二取代联苯接头组成的两亲链的共轭赋予了自组装特性。带有两亲性部分的全合成中等分子量 6-氮杂青蒿素6在环境温度下形成聚集体(约 200 nm),与N-苄基化氮杂青蒿素5相比,体外抗癌活性增加。
更新日期:2020-07-22
中文翻译:

带有两亲链的完全合成的 6-氮杂青蒿素会产生聚集体并表现出抗癌活性。
在青蒿素的 C6 位置安装一个氮有利于在环己烷部分(C 环)上添加一个功能单元。在这项研究中,由顺序连接的亲水低聚乙二醇、疏水烷基链、尿素和 4,4'-二取代联苯接头组成的两亲链的共轭赋予了自组装特性。带有两亲性部分的全合成中等分子量 6-氮杂青蒿素6在环境温度下形成聚集体(约 200 nm),与N-苄基化氮杂青蒿素5相比,体外抗癌活性增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号