当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intermolecular interactions and disorder in six isostructural celecoxib solvates.
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-06-27 , DOI: 10.1107/s2053229620008359 Andrew D Bond 1 , Changquan C Sun 2
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-06-27 , DOI: 10.1107/s2053229620008359 Andrew D Bond 1 , Changquan C Sun 2
Affiliation
|
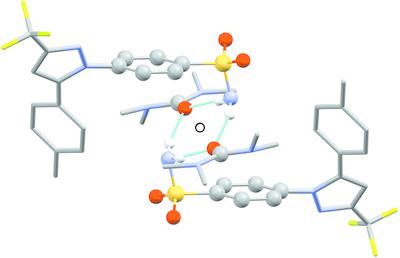
Six isostructural crystalline solvates of the active pharmaceutical ingredient celecoxib {4‐[5‐(4‐methylphenyl)‐3‐(trifluoromethyl)pyrazol‐1‐yl]benzenesulfonamide; C17H14F3N3O2S} are described, containing dimethylformamide (DMF, C3H7NO, 1 ), dimethylacetamide (DMA, C4H9NO, 2 ), N ‐methylpyrrolidin‐2‐one (NMP, C5H9NO, 3 ), tetramethylurea (TMU, C5H12N2O, 4 ), 1,3‐dimethyl‐3,4,5,6‐tetrahydropyrimidin‐2(1H )‐one (DMPU, C6H12N2O, 5 ) or dimethyl sulfoxide (DMSO, C2H6OS, 6 ). The host celecoxib structure contains one‐dimensional channel voids accommodating the solvent molecules, which accept hydrogen bonds from the NH2 groups of two celecoxib molecules. The solvent binding sites have local twofold rotation symmetry, which is consistent with the point symmetry of the solvent molecule in 4 and 5 , but introduces orientational disorder for the solvent molecules in 1 , 2 , 3 and 6 . Despite the isostructurality of 1 –6 , the unit‐cell volume and solvent‐accessible void space show significant variation. In particular, 4 and 5 show an enlarged and skewed unit cell, which can be attributed to a specific interaction between an N—CH3 group in the solvent molecule and the toluene group of celecoxib. Intermolecular interaction energies calculated using the PIXEL method show that the total interaction energy between the celecoxib and solvent molecules is broadly correlated with the molecular volume of the solvent, except in 6 , where the increased polarity of the S=O bond leads to greater overall stabilization compared to the similarly‐sized DMF molecule in 1 . In the structures showing disorder, the most stable orientations of the solvent molecules make C—H…O contacts to the S=O groups of celecoxib.
中文翻译:

六种同结构塞来昔布溶剂化物的分子间相互作用和紊乱。
活性药物成分塞来考昔的六种同构结晶溶剂化物{4-[5-(4-甲基苯基)-3-(三氟甲基)吡唑-1-基]苯磺酰胺;描述了C 17 H 14 F 3 N 3 O 2 S},含有二甲基甲酰胺(DMF,C 3 H 7 NO, 1 ),二甲基乙酰胺(DMA,C 4 H 9 NO, 2 ), N-甲基吡咯烷-2-酮( NMP, C 5 H 9 NO, 3 ), 四甲基脲 (TMU, C 5 H 12 N 2 O, 4 ), 1,3-二甲基-3,4,5,6-四氢嘧啶-2(1 H )-酮( DMPU,C 6 H 12 N 2 O, 5 )或二甲亚砜(DMSO,C 2 H 6 OS, 6 )。主体塞来昔布结构包含容纳溶剂分子的一维通道空隙,其接受来自两个塞来昔布分子的NH 2基团的氢键。溶剂结合位点具有局部双重旋转对称性,这与4和5中溶剂分子的点对称性一致,但引入了1、2、3和6中溶剂分子的取向无序。尽管具有1 – 6的同构结构,但晶胞体积和溶剂可及的空隙空间显示出显着的变化。特别地,图4和图5显示了放大且倾斜的晶胞,这可归因于溶剂分子中的N-CH 3基团与塞来昔布的甲苯基团之间的特定相互作用。 使用PIXEL方法计算的分子间相互作用能表明,塞来昔布和溶剂分子之间的总相互作用能与溶剂的分子体积广泛相关,但在6中除外,其中 S=O 键的极性增加导致整体稳定性更高与1中相似大小的DMF分子相比。在显示无序的结构中,溶剂分子最稳定的方向使CH…O与塞来昔布的S=O基团接触。
更新日期:2020-07-06
中文翻译:

六种同结构塞来昔布溶剂化物的分子间相互作用和紊乱。
活性药物成分塞来考昔的六种同构结晶溶剂化物{4-[5-(4-甲基苯基)-3-(三氟甲基)吡唑-1-基]苯磺酰胺;描述了C 17 H 14 F 3 N 3 O 2 S},含有二甲基甲酰胺(DMF,C 3 H 7 NO, 1 ),二甲基乙酰胺(DMA,C 4 H 9 NO, 2 ), N-甲基吡咯烷-2-酮( NMP, C 5 H 9 NO, 3 ), 四甲基脲 (TMU, C 5 H 12 N 2 O, 4 ), 1,3-二甲基-3,4,5,6-四氢嘧啶-2(1 H )-酮( DMPU,C 6 H 12 N 2 O, 5 )或二甲亚砜(DMSO,C 2 H 6 OS, 6 )。主体塞来昔布结构包含容纳溶剂分子的一维通道空隙,其接受来自两个塞来昔布分子的NH 2基团的氢键。溶剂结合位点具有局部双重旋转对称性,这与4和5中溶剂分子的点对称性一致,但引入了1、2、3和6中溶剂分子的取向无序。尽管具有1 – 6的同构结构,但晶胞体积和溶剂可及的空隙空间显示出显着的变化。特别地,图4和图5显示了放大且倾斜的晶胞,这可归因于溶剂分子中的N-CH 3基团与塞来昔布的甲苯基团之间的特定相互作用。 使用PIXEL方法计算的分子间相互作用能表明,塞来昔布和溶剂分子之间的总相互作用能与溶剂的分子体积广泛相关,但在6中除外,其中 S=O 键的极性增加导致整体稳定性更高与1中相似大小的DMF分子相比。在显示无序的结构中,溶剂分子最稳定的方向使CH…O与塞来昔布的S=O基团接触。











































 京公网安备 11010802027423号
京公网安备 11010802027423号