当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An explanation of the unusual strength of the hydrogen bond in small water clusters
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2020-06-27 , DOI: 10.1002/qua.26361 Shuman Li 1 , Alireza Azizi 1 , Steven R. Kirk 1 , Samantha Jenkins 1
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2020-06-27 , DOI: 10.1002/qua.26361 Shuman Li 1 , Alireza Azizi 1 , Steven R. Kirk 1 , Samantha Jenkins 1
Affiliation
|
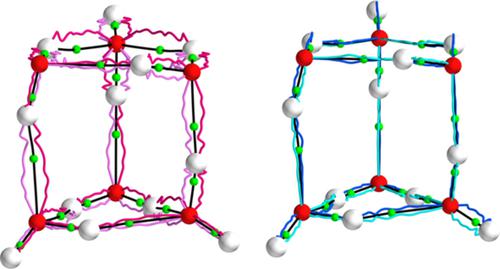
We seek to explain why the hydrogen bond possesses unusual strength in small water clusters that account for many of the complex behaviors of water. We have investigated and visualized the donation of covalent character from covalent (sigma) to hydrogen bonds by calculating the eigenvector coupling properties of quantum theory of atoms in molecules (QTAIM), stress tensor σ(r), and Ehrenfest Force F(r) on the F(r) molecular graph. The next‐generation three‐dimensional (3‐D) bond‐path framework sets are presented, and only the F(r) bond‐path framework sets reproduce the earlier finding on the coupling between covalent (sigma) and hydrogen bonds that possess a degree of covalent character. Exploration of the bond‐path between the covalent (sigma) and hydrogen bond's critical points provides an explanation for the previously obtained coupling results. The directional character of the covalent (sigma) and hydrogen bonds' 3‐D bond‐path framework sets for the F(r) explains differences found in the earlier results from QTAIM and the stress tensor σ(r).
中文翻译:

小水团簇中氢键异常强度的解释
我们试图解释为什么氢键在小水簇中具有不同寻常的强度,而小水簇解释了水的许多复杂行为。我们通过计算分子中原子的量子理论(QTAIM),应力张量σ(r)和Ehrenfest力F(r)的本征矢量耦合特性,研究并可视化了从共价(sigma)到氢键的共价特征的捐赠。所述˚F([R )分子图。提出了下一代三维(3-D)键合路径框架集,并且仅F(r)键路径框架集重现了较早的发现,即共价(sigma)与具有一定共价特征的氢键之间的偶联作用。对共价(σ)和氢键的临界点之间的键路径的探索为先前获得的偶联结果提供了解释。F(r)的共价键(sigma)和氢键的3-D键路径框架集的方向特征解释了QTAIM和应力张量σ(r)的早期结果中发现的差异。
更新日期:2020-08-29
中文翻译:

小水团簇中氢键异常强度的解释
我们试图解释为什么氢键在小水簇中具有不同寻常的强度,而小水簇解释了水的许多复杂行为。我们通过计算分子中原子的量子理论(QTAIM),应力张量σ(r)和Ehrenfest力F(r)的本征矢量耦合特性,研究并可视化了从共价(sigma)到氢键的共价特征的捐赠。所述˚F([R )分子图。提出了下一代三维(3-D)键合路径框架集,并且仅F(r)键路径框架集重现了较早的发现,即共价(sigma)与具有一定共价特征的氢键之间的偶联作用。对共价(σ)和氢键的临界点之间的键路径的探索为先前获得的偶联结果提供了解释。F(r)的共价键(sigma)和氢键的3-D键路径框架集的方向特征解释了QTAIM和应力张量σ(r)的早期结果中发现的差异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号