当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Removal of nickel from chemical plating waste solution through precipitation and production of microsized nickel hydroxide particles
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2020-06-27 , DOI: 10.1016/j.seppur.2020.117315 Tzu-Hsuan Tsai , Hao-Wei Chou , Yung-Fu Wu
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2020-06-27 , DOI: 10.1016/j.seppur.2020.117315 Tzu-Hsuan Tsai , Hao-Wei Chou , Yung-Fu Wu
|
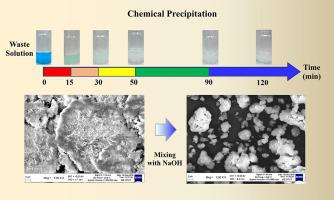
Chemical plating of nickel is widely used in wafer packaging. However, frequent renewal of plating baths generates a large amount of waste solution. When hazardous Ni ions are removed from waste solutions through chemical precipitation, the complexing agent in the solution hinders nickel removal and reduces the nickel hydroxide [Ni(OH)] precipitate size to approximately 0.5 μm. This study revealed that the precipitation rate of Ni(OH) can be controlled by the pH, temperature, and agitation. Furthermore, a Ni removal efficiency of more than 98% can be achieved. X-ray diffraction analysis revealed that the recovered particles included amorphous Ni(OH) and α-Ni(OH), indicating a high potential for energy conversion in nickel-based secondary batteries. Therefore, the proposed method can be used to sustain a circular economy by creating a Ni waste-to-energy cycle.
中文翻译:

通过沉淀和产生微米级氢氧化镍颗粒去除化学电镀废液中的镍
化学镀镍广泛应用于晶圆封装。然而,频繁更换镀液会产生大量废液。当通过化学沉淀从废液中去除有害镍离子时,溶液中的络合剂会阻碍镍的去除,并将氢氧化镍 [Ni(OH)] 沉淀物的尺寸减小至约 0.5 μm。这项研究表明,Ni(OH) 的沉淀速率可以通过 pH 值、温度和搅拌来控制。此外,Ni的去除效率可以达到98%以上。 X射线衍射分析表明,回收的颗粒包含非晶态Ni(OH)和α-Ni(OH),表明镍基二次电池具有很高的能量转换潜力。因此,所提出的方法可用于通过创建镍废物能源循环来维持循环经济。
更新日期:2020-06-27
中文翻译:

通过沉淀和产生微米级氢氧化镍颗粒去除化学电镀废液中的镍
化学镀镍广泛应用于晶圆封装。然而,频繁更换镀液会产生大量废液。当通过化学沉淀从废液中去除有害镍离子时,溶液中的络合剂会阻碍镍的去除,并将氢氧化镍 [Ni(OH)] 沉淀物的尺寸减小至约 0.5 μm。这项研究表明,Ni(OH) 的沉淀速率可以通过 pH 值、温度和搅拌来控制。此外,Ni的去除效率可以达到98%以上。 X射线衍射分析表明,回收的颗粒包含非晶态Ni(OH)和α-Ni(OH),表明镍基二次电池具有很高的能量转换潜力。因此,所提出的方法可用于通过创建镍废物能源循环来维持循环经济。











































 京公网安备 11010802027423号
京公网安备 11010802027423号