Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-06-27 , DOI: 10.1016/j.molliq.2020.113694 Anton S. Shalygin , Nikolai S. Nesterov , Sergei A. Prikhod'ko , Nikolai Y. Adonin , Oleg N. Martyanov , Sergei G. Kazarian
|
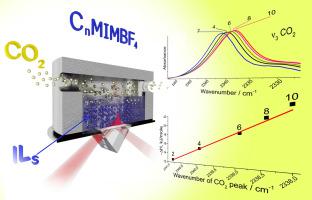
In this work, in situ ATR-FTIR spectroscopy was used to study the interaction of CO2 and a series of 1-alkyl-3-methylimidazolium tetrafluoroborate СnMIMBF4 (n = 2, 4, 6, 8, 10) ionic liquids. A detailed analysis of the infrared spectra acquired from ionic liquids and sorbed СО2 was performed as ionic liquids with different the alkyl chain lengths were subjected to changing pressures of СО2 and temperature. With a longer alkyl chain length, an increase in the shift of the CH stretching vibrations bands of alkyl groups and BF stretching vibrations of BF4− anions is observed during CO2 sorption. This indicates the disaggregation of alkyl chains and anions. There is a correlation with the position of the wavenumber of the ν3 asymmetric stretching CO2 band and the length of the alkyl chain. It was found that some of the CO2 adsorbed by ionic liquids does not interact with the ionic liquid but is “free” in the bulk of the alkyl chains. For the first time, ATR-FTIR spectroscopy was used to determine the thermodynamic parameters of CO2 sorption in ionic liquids. It was demonstrated that the values of enthalpy and entropy obtained by analysis of the ATR-FTIR spectra are consistent the data obtained by other methods. A correlation was found between the enthalpy of sorption of CO2 and the wavenumber of the ν3 band. This opens up the possibility of using CO2 as an IR-sensitive probe molecule to characterize the acid-base properties of ionic liquids and determine the enthalpy of CO2 sorption.
中文翻译:

CO的相互作用2与同系列С的Ñ MIMBF 4个的光谱特性,热力学参数和它们的相关性:研究原位ATR-FTIR光谱的离子液体
在这项工作中,原位ATR-FTIR光谱学用于研究CO的相互作用2和一系列的1-烷基-3-甲基咪唑鎓四氟硼酸盐С Ñ MIMBF 4(Ñ = 2,4,6,8,10)的离子液体。从离子液体中获取的红外光谱的详细分析和吸附СО 2具有不同的烷基链长进行变更的СО压力离子液体进行2和温度。具有较长烷基链的长度,增加了CH伸缩烷基和BF的BF伸缩振动的振动频带的移位4 -阴离子是CO过程中观察到2吸附。这表明烷基链和阴离子的分解。存在与ν的波数的位置的相关性3反对称伸缩CO 2频带和烷基链的长度。发现离子液体吸附的某些CO 2不与离子液体相互作用,但在大部分烷基链中“游离”。首次使用ATR-FTIR光谱法确定离子液体中CO 2吸附的热力学参数。结果表明,通过ATR-FTIR光谱分析获得的焓和熵值与通过其他方法获得的数据一致。发现CO 2的吸附焓之间存在相关性和ν的波数3频带。这开辟了使用CO 2作为IR敏感探针分子来表征离子液体的酸碱性质并确定CO 2吸附焓的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号