International Journal for Parasitology ( IF 3.7 ) Pub Date : 2020-06-27 , DOI: 10.1016/j.ijpara.2020.05.005 Desalegn Woldeyohannes Kifle 1 , Sujittra Chaiyadet 2 , Ashley J Waardenberg 1 , Ingrid Wise 3 , Martha Cooper 1 , Luke Becker 1 , Denise L Doolan 1 , Thewarach Laha 4 , Javier Sotillo 5 , Mark S Pearson 1 , Alex Loukas 1
|
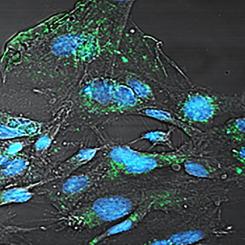
The ability of the parasitic blood fluke Schistosoma mansoni and other parasitic helminths to manipulate host biology is well recognised, but the mechanisms that underpin these phenomena are not well understood. An emerging paradigm is that helminths transfer their biological cargo to host cells by secretion of extracellular vesicles (EVs). Herein, we show that two populations of S. mansoni secreted EVs – exosome-like vesicles (ELVs) and microvesicles (MVs) – are actively internalised in two distinct human cell lines that reflect the resident cell types encountered by the parasite in vivo: human umbilical vein endothelial cells (HUVECs) and THP-1 monocytes. RNA-sequencing of HUVECs co-cultured with S. mansoni ELVs compared with untreated HUVECs revealed differential expression of genes associated with intravascular parasitism, including vascular endothelial contraction, coagulation, arachidonic acid metabolism and immune cell trafficking and signalling. Finally, we show that antibodies raised against recombinant tetraspanin (TSP) proteins from the surface of S. mansoni EVs significantly blocked EV uptake by both HUVECs and THP-1 monocytes whereas pre-immunisation antibodies did not. To our knowledge, this is the first evidence demonstrating the internalisation of secreted EVs from any helminth into vascular endothelial cells, providing novel insight into the potential mechanisms underlying host-schistosome interactions. The ability of anti-TSP antibodies to block vesicle uptake by host target cells further supports the potential of TSPs as promising antigens for an anti-fluke vaccine. It also suggests a potential mechanism whereby the current candidate human schistosomiasis vaccine, Sm-TSP-2, exerts its protective effect in animal models.
中文翻译:

曼氏血吸虫胞外小泡被人内皮细胞和单核细胞系摄取并影响血管内皮细胞基因表达。
寄生血吸虫曼氏血吸虫和其他寄生蠕虫操纵宿主生物学的能力已得到广泛认可,但支撑这些现象的机理尚不十分清楚。新兴的范例是蠕虫通过分泌细胞外囊泡(EVs)将其生物负载转移到宿主细胞。本文中,我们显示了曼氏葡萄球菌分泌的两个EV群体(外泌体样囊泡(ELV)和微囊泡(MV))在两种截然不同的人类细胞系中被主动内化,这些细胞系反映了体内寄生虫遇到的常驻细胞类型:人类脐静脉内皮细胞(HUVEC)和THP-1单核细胞。与曼氏链球菌共培养的HUVEC的RNA测序与未处理的HUVEC相比,ELVs显示与血管内寄生虫相关的基因差异表达,包括血管内皮收缩,凝血,花生四烯酸代谢以及免疫细胞运输和信号传导。最后,我们显示了从曼氏葡萄球菌表面引发的针对重组四跨膜蛋白(TSP)蛋白的抗体EV显着阻止了HUVEC和THP-1单核细胞摄取EV,而免疫前抗体却没有。据我们所知,这是第一个证明分泌的EV从任何蠕虫进入血管内皮细胞的内在化作用的第一个证据,为宿主-血吸虫相互作用的潜在机制提供了新颖的见解。抗TSP抗体阻止宿主靶细胞摄取囊泡的能力进一步支持了TSP作为抗流感疫苗的有希望的抗原的潜力。它还暗示了一种潜在的机制,通过该机制,当前的候选人类血吸虫病疫苗Sm -TSP-2在动物模型中发挥了保护作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号