Dyes and Pigments ( IF 4.1 ) Pub Date : 2020-06-27 , DOI: 10.1016/j.dyepig.2020.108628 Jelena Lađarević , Dušan Mijin , Liudmil Antonov
|
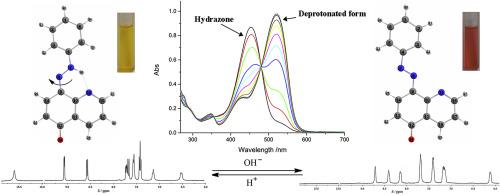
The tautomerism of 8-(phenyldiazenyl)quinolin-5-ol has been studied by a combination of theoretical (DFT calculations) and experimental (UV–Vis and NMR) methods. The detailed study of neutral molecules has shown that the hydrazone tautomeric form, stabilized by an intramolecular N–H⋯N hydrogen bond, is solely present in most of the solvents. In strong proton acceptor solvents, besides the dominant hydrazone form, the deprotonated form also appears. Solvent effects on the absorption maxima of the hydrazone form are interpreted by the linear solvation energy relationship concept, using Kamlet-Taft and Catalán models. Upon deprotonation, a substantial structural transformation is observed in the studied compound leading to a slow rotation around the Cquin-N bond. The process, as monitored by 1H NMR, is strongly solvent assisted and facilitated by proton acceptor solvents. Consequently, the investigated dye could be considered as a base-activated rotary switch.
中文翻译:

8-(苯基二氮烯基)喹啉-5-醇中的互变异构现象:pH活化旋转开关的尝试
已经通过理论(DFT计算)和实验(UV-Vis和NMR)方法的组合研究了8-(苯基二氮烯基)喹啉-5-醇的互变异构现象。对中性分子的详细研究表明,by互变异构体形式(通过分子内N–H⋯N氢键稳定)仅存在于大多数溶剂中。在强质子受体溶剂中,除主要的form形式外,还会出现去质子化的形式。使用Kamlet-Taft和Catalán模型,线性溶剂化能量关系概念解释了溶剂对on形式最大吸收的影响。去质子化后,在所研究的化合物中观察到实质性的结构转变,导致围绕Cquin-N键的缓慢旋转。由1监控的过程1 H NMR由质子受体溶剂强烈地辅助和促进。因此,所研究的染料可以被认为是碱基激活的旋转开关。









































 京公网安备 11010802027423号
京公网安备 11010802027423号