当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binding ability of arsenate towards Cu2+ and Zn2+: thermodynamic behavior and simulation under natural water conditions.
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2020-06-26 , DOI: 10.1039/d0em00136h Ottavia Giuffrè 1 , Donatella Aiello 2 , Donatella Chillè 1 , Anna Napoli 2 , Claudia Foti 1
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2020-06-26 , DOI: 10.1039/d0em00136h Ottavia Giuffrè 1 , Donatella Aiello 2 , Donatella Chillè 1 , Anna Napoli 2 , Claudia Foti 1
Affiliation
|
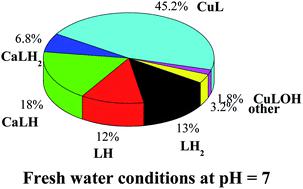
A study on the sequestering ability between arsenate, AsO43−, and Cu2+ and Zn2+ in aqueous solution is reported. The results of the elaboration of potentiometric data include only species with 1 : 1 metal to ligand ratio for Cu2+–arsenate system, namely CuLH2, CuLH, CuL, and CuLOH (L = AsO43−). For the Zn2+–arsenate system, a speciation model with only two species with both 1 : 1 and 1 : 2 metal to ligand ratios was obtained, namely ML and ML2. Spectrophotometric titrations were also employed in the study of the Cu2+–AsO43− system, and the results of the analysis of experimental data fully confirmed potentiometric ones. The potentiometric titrations were performed under different conditions of temperature (288.15 ≤ T/K ≤ 310.15, at I = 0.15 mol L−1) and ionic strength (0.15 ≤ I/mol L−1 ≤ 1 in NaCl). The dependence of formation constants of the complex species on ionic strength and temperature was also evaluated, as well as the enthalpy and entropy change values were obtained. Laser desorption mass spectrometry (LD MS) and tandem mass spectrometry (MS/MS) were exploited to confirm Cu2+–AsO43− and Zn2+–AsO43− complex formation and to determine both their composition and structural characteristics. Simulation of speciation profiles under natural water conditions was performed. The sequestering ability of arsenate towards Cu2+ and Zn2+ was quantified under different conditions of pH, temperature and ionic strength, typical of several natural waters. Examples of arsenate distribution under seawater and freshwater conditions were reported.
中文翻译:

砷酸盐对Cu2 +和Zn2 +的结合能力:在自然水条件下的热力学行为和模拟。
报道了关于砷酸盐,AsO 4 3-和Cu 2+和Zn 2+在水溶液中的螯合能力的研究。电位数据的精炼结果仅包括Cu 2 +-砷酸盐系统的金属与配体比率为1:1的物种,即CuLH 2,CuLH,CuL和CuLOH(L = AsO 4 3−)。对于Zn 2 +-砷酸盐体系,获得了只有两种物种的金属和配体比率为1:1和1:2的物种形成模型,即ML和ML 2。分光光度滴定法也用于研究Cu 2+ -AsO 4 3-系统,实验数据的分析结果完全证实了电位计。的电位滴定温度的不同条件下进行(288.15≤ Ť / K≤310.15,在我= 0.15摩尔大号-1)和离子强度(0.15≤我/摩尔大号-1在NaCl≤1)。还评估了复合物种类的形成常数对离子强度和温度的依赖性,并获得了焓和熵变值。利用激光解吸质谱(LD MS)和串联质谱(MS / MS)来确定Cu 2+ -AsO 4 3-和Zn 2+ -AsO 4 3-并确定它们的组成和结构特征。在天然水条件下进行了形态分析。在几种天然水的典型pH,温度和离子强度的不同条件下,量化了砷酸盐对Cu 2+和Zn 2+的螯合能力。报告了在海水和淡水条件下砷分布的实例。
更新日期:2020-08-19
中文翻译:

砷酸盐对Cu2 +和Zn2 +的结合能力:在自然水条件下的热力学行为和模拟。
报道了关于砷酸盐,AsO 4 3-和Cu 2+和Zn 2+在水溶液中的螯合能力的研究。电位数据的精炼结果仅包括Cu 2 +-砷酸盐系统的金属与配体比率为1:1的物种,即CuLH 2,CuLH,CuL和CuLOH(L = AsO 4 3−)。对于Zn 2 +-砷酸盐体系,获得了只有两种物种的金属和配体比率为1:1和1:2的物种形成模型,即ML和ML 2。分光光度滴定法也用于研究Cu 2+ -AsO 4 3-系统,实验数据的分析结果完全证实了电位计。的电位滴定温度的不同条件下进行(288.15≤ Ť / K≤310.15,在我= 0.15摩尔大号-1)和离子强度(0.15≤我/摩尔大号-1在NaCl≤1)。还评估了复合物种类的形成常数对离子强度和温度的依赖性,并获得了焓和熵变值。利用激光解吸质谱(LD MS)和串联质谱(MS / MS)来确定Cu 2+ -AsO 4 3-和Zn 2+ -AsO 4 3-并确定它们的组成和结构特征。在天然水条件下进行了形态分析。在几种天然水的典型pH,温度和离子强度的不同条件下,量化了砷酸盐对Cu 2+和Zn 2+的螯合能力。报告了在海水和淡水条件下砷分布的实例。







































 京公网安备 11010802027423号
京公网安备 11010802027423号