当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of Some Aminosugars via Catalytic Aminohydroxylation of Protected 2,3-Unsaturated d-Gluco- and d-Galacto-2-hexenopyranosides.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-26 , DOI: 10.1021/acs.joc.0c01170 Ippei Fukuhara 1 , Ryosuke Matsubara 1 , Masahiko Hayashi 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-26 , DOI: 10.1021/acs.joc.0c01170 Ippei Fukuhara 1 , Ryosuke Matsubara 1 , Masahiko Hayashi 1
Affiliation
|
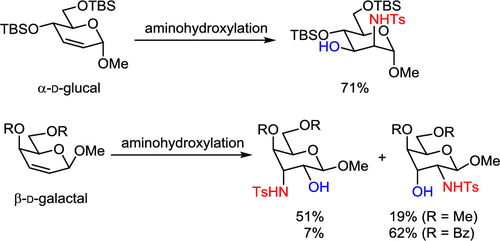
The aminohydroxylation of methyl 4,6-di-O-(tert-butyldimethylsilyl)-2,3-unsaturated α-d-glucopyranoside proceeds in the presence of chloramine-T, OsO4 (4 mol %), (DHQ)2PHAL (5 mol %), and triethylbenzylammonium chloride (TEBAC) in both a stereoselective and a regioselective manner to produce protected methyl α-d-mannosamide as the sole product. In contrast, the reaction of methyl 2,3-unsaturated β-d-galactopyranoside under the same conditions produced a mixture of regioisomers, although the stereochemistry was perfectly controlled. The regioisomeric ratio was dependent on the nature of the protecting group and the ligand used.
中文翻译:

通过保护的2,3-不饱和d-葡糖醇和d-Galacto-2-hexenopyranosides的催化氨基羟化反应选择性合成某些氨基糖。
在氯胺-T,OsO 4(4 mol%),(DHQ)2 PHAL的存在下,进行甲基4,6-二-O-(叔丁基二甲基甲硅烷基)-2,3-不饱和α- d-吡喃葡萄糖苷的氨基羟化反应(5mol%)和三乙基苄基氯化铵(TEBAC)以立体选择性和区域选择性两种方式产生作为唯一产物的受保护的甲基α- d-甘露糖酰胺。相反,在2,3-不饱和甲基β- d-吡喃半乳糖苷在相同条件下的反应产生了区域异构体的混合物,尽管立体化学得到了很好的控制。区域异构体的比例取决于保护基和所用配体的性质。
更新日期:2020-07-17
中文翻译:

通过保护的2,3-不饱和d-葡糖醇和d-Galacto-2-hexenopyranosides的催化氨基羟化反应选择性合成某些氨基糖。
在氯胺-T,OsO 4(4 mol%),(DHQ)2 PHAL的存在下,进行甲基4,6-二-O-(叔丁基二甲基甲硅烷基)-2,3-不饱和α- d-吡喃葡萄糖苷的氨基羟化反应(5mol%)和三乙基苄基氯化铵(TEBAC)以立体选择性和区域选择性两种方式产生作为唯一产物的受保护的甲基α- d-甘露糖酰胺。相反,在2,3-不饱和甲基β- d-吡喃半乳糖苷在相同条件下的反应产生了区域异构体的混合物,尽管立体化学得到了很好的控制。区域异构体的比例取决于保护基和所用配体的性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号