当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modifying Phosphorus(III) Substituents to Activate Remote Ligand-Centered Reactivity in Triaminoborane Ligands
Organometallics ( IF 2.5 ) Pub Date : 2020-06-25 , DOI: 10.1021/acs.organomet.0c00321 Kyounghoon Lee 1 , Johnathan D. Culpepper 1 , Riffat Parveen 2 , Dale C. Swenson 1 , Bess Vlaisavljevich 2 , Scott R. Daly 1
Organometallics ( IF 2.5 ) Pub Date : 2020-06-25 , DOI: 10.1021/acs.organomet.0c00321 Kyounghoon Lee 1 , Johnathan D. Culpepper 1 , Riffat Parveen 2 , Dale C. Swenson 1 , Bess Vlaisavljevich 2 , Scott R. Daly 1
Affiliation
|
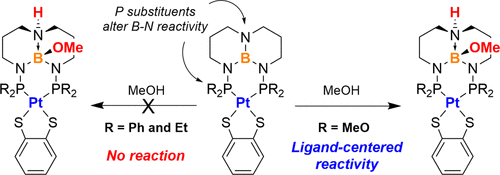
Triaminoborane-bridged diphosphorus ligands called TBDPhos can undergo cooperative ligand-centered reactions at the 1,8,10,9-triazaboradecalin (TBD) backbone while bound to metals, but the factors that control this reactivity are not well understood. Previous studies showed that TBDPhos reactivity is highly sensitive to phosphorus substituents, ancillary ligands, and metal coordination environment despite these changes being significantly removed from the reactive N–B bond. Here we describe how exchanging phenyl and ethyl substituents attached to phosphorus with methoxy turns on ligand-centered reactions in otherwise unreactive (TBDPhos)Pt(S2C6H4) and (TBDPhos)Mo(CO)4 complexes. The synthesis and characterization of the complexes are described alongside comparative reactivity studies using MeOH as a simple but effective test substrate. Complementary density functional theory (DFT) calculations on an expanded series of (TBDPhos)Pt(S2C6H4) complexes corroborate the experimental results and show that both size and electronic properties of the phosphorus substituents can significantly influence the Gibbs free energy of reaction at the remote ligand site. These results demonstrate how seemingly ancillary ligand modifications can levy significant control over ligand-centered reactivity in TBDPhos complexes, which may be relevant when considering the design of other chemically reactive ligands with Lewis acidic functional groups.
中文翻译:

修改磷(III)取代基以激活三氨基硼烷配体中远程配体中心反应性
三氨基硼烷桥联的二磷配体称为TBDPhos可以在与金属结合时在1,8,10,9-三氮硼硼烷(TBD)骨架上进行以配体为中心的协同反应,但控制这种反应性的因素尚不清楚。先前的研究表明,TBDPhos的反应性对磷取代基,辅助配体和金属配位环境非常敏感,尽管这些变化已从活性N-B键中明显除去。在这里,我们描述了如何用甲氧基交换连接到磷上的苯基和乙基取代基如何打开在未反应的(TBDPhos)Pt(S 2 C 6 H 4)和(TBDPhos)Mo(CO)4中以配体为中心的反应复合体。配合物的合成和表征与使用MeOH作为简单但有效的测试底物的比较反应性研究一起进行了描述。(TBDPhos)Pt(S 2 C 6 H 4)的扩展系列的互补密度泛函理论(DFT)计算)配合物证实了实验结果,表明磷取代基的大小和电子性质都可以显着影响远端配体位点的吉布斯反应自由能。这些结果表明,看似辅助的配体修饰如何能够对TBDPhos络合物中以配体为中心的反应性进行重要控制,这在考虑设计具有路易斯酸性官能团的其他化学反应性配体时可能是相关的。
更新日期:2020-07-13
中文翻译:

修改磷(III)取代基以激活三氨基硼烷配体中远程配体中心反应性
三氨基硼烷桥联的二磷配体称为TBDPhos可以在与金属结合时在1,8,10,9-三氮硼硼烷(TBD)骨架上进行以配体为中心的协同反应,但控制这种反应性的因素尚不清楚。先前的研究表明,TBDPhos的反应性对磷取代基,辅助配体和金属配位环境非常敏感,尽管这些变化已从活性N-B键中明显除去。在这里,我们描述了如何用甲氧基交换连接到磷上的苯基和乙基取代基如何打开在未反应的(TBDPhos)Pt(S 2 C 6 H 4)和(TBDPhos)Mo(CO)4中以配体为中心的反应复合体。配合物的合成和表征与使用MeOH作为简单但有效的测试底物的比较反应性研究一起进行了描述。(TBDPhos)Pt(S 2 C 6 H 4)的扩展系列的互补密度泛函理论(DFT)计算)配合物证实了实验结果,表明磷取代基的大小和电子性质都可以显着影响远端配体位点的吉布斯反应自由能。这些结果表明,看似辅助的配体修饰如何能够对TBDPhos络合物中以配体为中心的反应性进行重要控制,这在考虑设计具有路易斯酸性官能团的其他化学反应性配体时可能是相关的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号