当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Distinctive Viewpoint on the Rapid Dissolution Mechanism of α-Chitin in Aqueous Potassium Hydroxide–Urea Solution at Low Temperatures
Macromolecules ( IF 5.1 ) Pub Date : 2020-06-26 , DOI: 10.1021/acs.macromol.0c00945 Junchao Huang 1 , Yi Zhong 1 , Lina Zhang 1, 2 , Jie Cai 1, 2
Macromolecules ( IF 5.1 ) Pub Date : 2020-06-26 , DOI: 10.1021/acs.macromol.0c00945 Junchao Huang 1 , Yi Zhong 1 , Lina Zhang 1, 2 , Jie Cai 1, 2
Affiliation
|
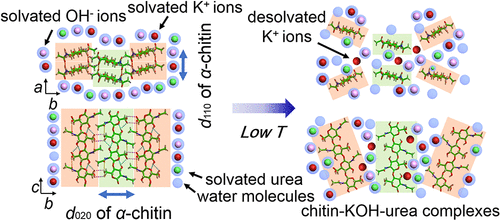
To develop a green solvent for chitin dissolution, the most fundamental aspects of its mechanism must be elucidated. In this work, an aqueous KOH/urea solution was utilized for the rapid dissolution of α-chitin without performing freeze–thaw cycles. It was found that the mechanism of α-chitin dissolution involved not only the fast expansion of its crystalline regions and hierarchical structure but also direct and strong interactions between K+ ions and chitin carbonyl oxygens. The relatively high flexibility and deformability of the K+ hydration shells allowed fast exchange between water molecules and carbonyl oxygens, which induced the cleavage of strong inter- and intramolecular hydrogen bonds, resulting in the rapid swelling and destruction of the chitin crystal and dissolution of α-chitin. Moreover, the additional urea mitigated the hydrophobicity of chitin chains, which prevented self-aggregation and increased the mobility of chitin chains. The described α-chitin dissolution mechanism can help achieve a better understanding of biomacromolecule dissolution.
中文翻译:

低温下α-几丁质在氢氧化钾-尿素水溶液中的快速溶解机理的独特观点
为了开发一种绿色的几丁质溶解溶剂,必须阐明其机理的最基本方面。在这项工作中,使用KOH /尿素水溶液可快速溶解α-几丁质而不进行冻融循环。发现α-几丁质溶解的机理不仅涉及其晶体区域和层次结构的快速膨胀,而且还涉及K +离子与几丁质羰基氧之间的直接和强烈的相互作用。K +相对较高的柔韧性和可变形性水合壳使水分子和羰基氧之间能够快速交换,从而引起强分子间和分子内氢键的裂解,导致几丁质晶体迅速溶胀和破坏,α-几丁质溶解。此外,额外的尿素减轻了几丁质链的疏水性,阻止了自身聚集并增加了几丁质链的迁移率。所述的α-几丁质溶解机制可以帮助更好地理解生物大分子的溶解。
更新日期:2020-07-14
中文翻译:

低温下α-几丁质在氢氧化钾-尿素水溶液中的快速溶解机理的独特观点
为了开发一种绿色的几丁质溶解溶剂,必须阐明其机理的最基本方面。在这项工作中,使用KOH /尿素水溶液可快速溶解α-几丁质而不进行冻融循环。发现α-几丁质溶解的机理不仅涉及其晶体区域和层次结构的快速膨胀,而且还涉及K +离子与几丁质羰基氧之间的直接和强烈的相互作用。K +相对较高的柔韧性和可变形性水合壳使水分子和羰基氧之间能够快速交换,从而引起强分子间和分子内氢键的裂解,导致几丁质晶体迅速溶胀和破坏,α-几丁质溶解。此外,额外的尿素减轻了几丁质链的疏水性,阻止了自身聚集并增加了几丁质链的迁移率。所述的α-几丁质溶解机制可以帮助更好地理解生物大分子的溶解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号