当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Synthesis of a [2.2]Paracyclophane‐based Planar Chiral Dirhodium Catalyst and its Applications in Cyclopropanation Reaction of Vinylarenes with α‐Methyl‐α‐Diazo Esters
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-25 , DOI: 10.1002/adsc.202000512 Christoph Zippel 1 , Zahid Hassan 1, 2 , Martin Nieger 3 , Stefan Bräse 1, 2, 4
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-25 , DOI: 10.1002/adsc.202000512 Christoph Zippel 1 , Zahid Hassan 1, 2 , Martin Nieger 3 , Stefan Bräse 1, 2, 4
Affiliation
|
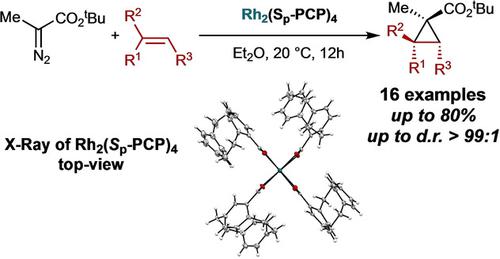
A planar chiral dirhodium paddlewheel complex Rh2(Sp−PCP)4 based on the [2.2]paracyclophane has been synthesized for the challenging cyclopropanation of venylarene derivatives with tert‐butyl α‐diazo propionates. The homobimetallic rhodium catalyst relies on the high steric demand and rigidity of [2.2]paracyclophane that favors the cyclopropanation of 1‐aryl substituted, 1,1‐disubstituted and benzannulated alkenes over β‐hydride migration at room temperature with high diastereoselectivity.
中文翻译:

[2.2]基于环芳烷的平面手性铱催化剂的设计,合成及其在乙烯基芳烃与α-甲基-α-重氮酯的环丙烷化反应中的应用
合成了一种基于[2.2]对环环糊精的平面手性ho铑配合物Rh 2(S p -PCP)4,用于具有挑战性的丙烯芳烃衍生物与叔丁基α-重氮丙酸酯的环丙烷化。均双金属铑催化剂依赖于[2.2]对环环烷的高空间需求和刚性,后者在室温下具有较高的非对映选择性,有利于1-芳基取代的,1,1-二取代的和苯并环化的烯烃的环丙烷化,而不是β-氢化物的迁移。
更新日期:2020-08-19
中文翻译:

[2.2]基于环芳烷的平面手性铱催化剂的设计,合成及其在乙烯基芳烃与α-甲基-α-重氮酯的环丙烷化反应中的应用
合成了一种基于[2.2]对环环糊精的平面手性ho铑配合物Rh 2(S p -PCP)4,用于具有挑战性的丙烯芳烃衍生物与叔丁基α-重氮丙酸酯的环丙烷化。均双金属铑催化剂依赖于[2.2]对环环烷的高空间需求和刚性,后者在室温下具有较高的非对映选择性,有利于1-芳基取代的,1,1-二取代的和苯并环化的烯烃的环丙烷化,而不是β-氢化物的迁移。







































 京公网安备 11010802027423号
京公网安备 11010802027423号