当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cinchona Squaramide‐Catalyzed Intermolecular Desymmetrization of 1,3‐Diketones Leading to Chiral 1,4‐Dihydropyridines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-25 , DOI: 10.1002/adsc.202000455 Maciej Dajek 1 , Agnieszka Pruszczyńska 1 , Krzysztof A. Konieczny 1 , Rafał Kowalczyk 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-25 , DOI: 10.1002/adsc.202000455 Maciej Dajek 1 , Agnieszka Pruszczyńska 1 , Krzysztof A. Konieczny 1 , Rafał Kowalczyk 1
Affiliation
|
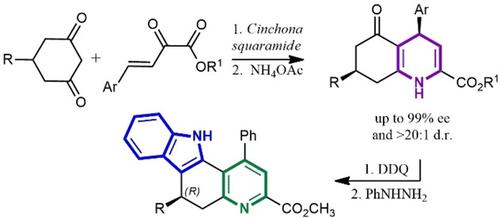
Addition of prochiral cyclic 1,3‐diketones to Michael acceptors applying bifunctional Cinchona‐derived squaramides resulted in chiral adducts with stereoselectivities of up to 99% ee and allowed for desymmetrization of the nucleophile. These labile hemiacetal intermediates were transformed to new 1,4‐dihydropyridines with high diastereoselectivities and no erosion of optical purity. Their further oxidation to pyridine followed by Fisher indolization provided chiral pyridine‐indoles.
中文翻译:

Cinchona Squaramide催化的1,3-二酮的分子间脱对称导致手性1,4-二氢吡啶
在使用双官能金鸡纳衍生的方酰胺的迈克尔受体上添加前手性环状1,3-二酮会导致手性加合物的立体选择性高达99%ee,并允许亲核试剂脱对称。这些不稳定的半缩醛中间体被转化为新的1,4-二氢吡啶,具有高非对映选择性,且不会破坏光学纯度。它们进一步氧化为吡啶,然后进行费舍尔吲哚化,提供了手性吡啶吲哚。
更新日期:2020-06-25
中文翻译:

Cinchona Squaramide催化的1,3-二酮的分子间脱对称导致手性1,4-二氢吡啶
在使用双官能金鸡纳衍生的方酰胺的迈克尔受体上添加前手性环状1,3-二酮会导致手性加合物的立体选择性高达99%ee,并允许亲核试剂脱对称。这些不稳定的半缩醛中间体被转化为新的1,4-二氢吡啶,具有高非对映选择性,且不会破坏光学纯度。它们进一步氧化为吡啶,然后进行费舍尔吲哚化,提供了手性吡啶吲哚。











































 京公网安备 11010802027423号
京公网安备 11010802027423号