当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu Atomic Chain Supported on Graphene Nanoribbon for Effective Conversion of CO2 to Ethanol.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-07-17 , DOI: 10.1002/cphc.202000476 Haoming Shen 1 , Qiang Sun 1, 2
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-07-17 , DOI: 10.1002/cphc.202000476 Haoming Shen 1 , Qiang Sun 1, 2
Affiliation
|
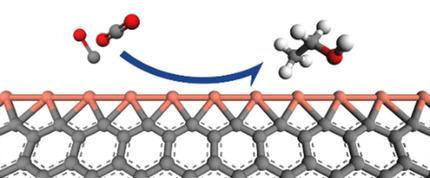
Cu catalysts are well‐known for their good performance in CO2 conversion. Compared to CO and CH4 production, C2 products have higher volumetric energy densities and are more valuable in industrial applications. In this work, we screened the catalytic ability of C2 production on several 1D Cu atomic chain structures and find that Cu edge‐decorated zigzag graphene nanoribbons (Cu−ZGNR) are capable of catalyzing CO2 conversion to ethanol, and CH3CH2OH is the main C2 product with a maximum free energy change of 0.60 eV. The planar tetracoordinate carbon structures in Cu‐ZGNR provide unique chemical bonding features for catalytic reaction on the Cu atoms. Detailed mechanism analyses with transition states search show that CO* dimerization is favored against CHO* formation in the reaction. By adjusting the CO* coverage, the selectivity of the C2 product can be enhanced owing to less pronounced steric effects for COCHO*, which is feasible under experimental conditions. This study expands the catalyst family for C2 products from CO2 based on nano carbon structures with new features.
中文翻译:

负载在石墨烯纳米带上的Cu原子链可有效将CO2转化为乙醇。
铜催化剂以其在CO 2转化中的良好性能而闻名。与CO和CH 4生产相比,C 2产品具有更高的体积能量密度,在工业应用中更有价值。在这项工作中,我们筛选了在一些一维Cu原子链结构上产生C 2的催化能力,发现Cu边缘修饰的之字形石墨烯纳米带(Cu-ZGNR)能够催化CO 2转化为乙醇和CH 3 CH 2。 OH是主要的C 2产品的最大自由能变化为0.60 eV。Cu-ZGNR中的平面四配位碳结构为铜原子上的催化反应提供了独特的化学键合功能。用过渡态搜索进行详细的机理分析表明,CO *二聚反应有利于反应中CHO *的形成。通过调整CO *的覆盖范围,由于对COCHO *的空间效应较不明显,因此可以提高C 2产物的选择性,这在实验条件下是可行的。这项研究扩展了具有新功能的基于纳米碳结构的CO 2的C 2产物的催化剂系列。
更新日期:2020-07-17
中文翻译:

负载在石墨烯纳米带上的Cu原子链可有效将CO2转化为乙醇。
铜催化剂以其在CO 2转化中的良好性能而闻名。与CO和CH 4生产相比,C 2产品具有更高的体积能量密度,在工业应用中更有价值。在这项工作中,我们筛选了在一些一维Cu原子链结构上产生C 2的催化能力,发现Cu边缘修饰的之字形石墨烯纳米带(Cu-ZGNR)能够催化CO 2转化为乙醇和CH 3 CH 2。 OH是主要的C 2产品的最大自由能变化为0.60 eV。Cu-ZGNR中的平面四配位碳结构为铜原子上的催化反应提供了独特的化学键合功能。用过渡态搜索进行详细的机理分析表明,CO *二聚反应有利于反应中CHO *的形成。通过调整CO *的覆盖范围,由于对COCHO *的空间效应较不明显,因此可以提高C 2产物的选择性,这在实验条件下是可行的。这项研究扩展了具有新功能的基于纳米碳结构的CO 2的C 2产物的催化剂系列。











































 京公网安备 11010802027423号
京公网安备 11010802027423号