当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of N‐Acylimidazoles in the Claisen Condensation Reaction
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-06-26 , DOI: 10.1002/slct.202001944 Haiyuan Quan 1 , Liuyang Wang 1 , Zhinan Wang 1 , Xiangdong Mei 1 , Jun Ning 1 , Dongmei She 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-06-26 , DOI: 10.1002/slct.202001944 Haiyuan Quan 1 , Liuyang Wang 1 , Zhinan Wang 1 , Xiangdong Mei 1 , Jun Ning 1 , Dongmei She 1
Affiliation
|
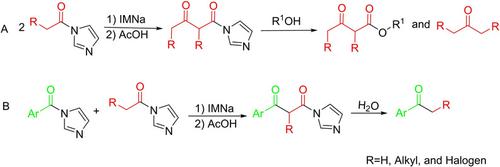
The Claisen condensation reaction is a classical method used for the formation of C−C bonds. Homo‐ and cross‐Claisen condensation reactions are usually carried out using a Ti(IV)‐based reagent at ultra‐low‐temperatures. In this report, intermediate β‐keto acyl imidazoles were synthesised using a sodium imidazolide‐catalysed homo‐ and cross‐condensation reaction of N ‐acyl imidazoles performed under mild conditions and subsequently used to prepare a range of β‐keto esters and ketones via alcoholysis or hydrolysis. This method is particularly suitable for the synthesis of isotopically‐labelled acetylacetic ester compounds. N ‐acyl imidazole compounds are useful reactants for the Claisen condensation reaction.
中文翻译:

N-酰基咪唑在克莱森缩合反应中的应用
克莱森缩合反应是用于形成C-C键的经典方法。均相和交叉克莱森缩合反应通常使用基于Ti(IV)的试剂在超低温下进行。在本报告中,使用咪唑啉钠催化的N-酰基咪唑的均相和交叉缩合反应,在温和的条件下进行合成,合成了中间的β-酮酰基咪唑,随后用于通过醇解法制备一系列β-酮酯和酮或水解。该方法特别适合于同位素标记的乙酰乙酸酯化合物的合成。N酰基咪唑化合物可用于克莱森缩合反应。
更新日期:2020-06-26
中文翻译:

N-酰基咪唑在克莱森缩合反应中的应用
克莱森缩合反应是用于形成C-C键的经典方法。均相和交叉克莱森缩合反应通常使用基于Ti(IV)的试剂在超低温下进行。在本报告中,使用咪唑啉钠催化的N-酰基咪唑的均相和交叉缩合反应,在温和的条件下进行合成,合成了中间的β-酮酰基咪唑,随后用于通过醇解法制备一系列β-酮酯和酮或水解。该方法特别适合于同位素标记的乙酰乙酸酯化合物的合成。N酰基咪唑化合物可用于克莱森缩合反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号