Tetrahedron ( IF 2.1 ) Pub Date : 2020-06-26 , DOI: 10.1016/j.tet.2020.131353 Xiao Zhu , Zhi-Qiang Zhu , Dong Guo , Shan Liu , Jiu-Jian Ji , Juan Tang , En Yuan , Zong-Bo Xie , Zhang-Gao Le
|
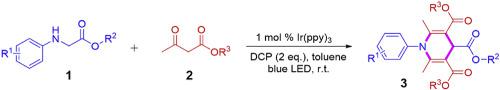
Visible light photocatalytic cascade cyclization reaction between glycine derivatives and β-ketoesters using Ir (ppy)3 as a catalyst and dicumyl peroxide (DCP) as an oxidant was described. A series of N-aryl glycine esters proceeded the cyclization smoothly with β-ketoesters at room temperature, affording the desired 1,4-dihydropyridines (1,4-DHPs) in satisfactory yields. A possible mechanism for the cascade cyclization reaction by visible light photoredox catalysis was also proposed. This protocol not only provides an efficient and convenient approach to synthetize various 1,4-dihydropyridines, but also has potential utilities for the construction of bioactive molecules.
中文翻译:

可见光光氧化还原催化β-酮酸酯对甘氨酸衍生物的级联环化反应,以取代多取代的1,4-二氢吡啶
描述了使用Ir(ppy)3作为催化剂和过氧化二枯基(DCP)作为氧化剂的甘氨酸衍生物与β-酮酸酯之间的可见光光催化级联环化反应。一系列N-芳基甘氨酸酯与β-酮酸酯在室温下顺利进行环化反应,以令人满意的产率提供所需的1,4-二氢吡啶(1,4-DHPs)。还提出了可见光氧化还原催化级联环化反应的可能机理。该协议不仅为合成各种1,4-二氢吡啶提供了一种高效便捷的方法,而且还具有潜在的实用性来构建生物活性分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号