Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-06-26 , DOI: 10.1016/j.jhazmat.2020.123324 Xuan Guo 1 , Yong Liu 2 , Jianlong Wang 3
|
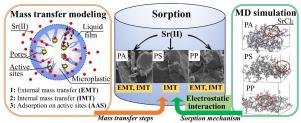
Microplastics (MPs) are becoming ubiquitous pollutants in the global environments, which can potentially sorb metals ions in aquatic environments, causing adverse consequences. The interaction between Sr2+ and MPs, and the involved mechanisms have not been studied. Here we investigated the sorption behaviors of Sr2+ by polyamide (PA), polystyrene (PS), and polypropylene (PP). Three phenomenological mathematical models were developed and applied to describe the rate-limiting step in the sorption process. The molecular dynamic (MD) simulation was also conducted to investigate the sorption mechanism. The results showed that the optimum isotherm was presented by the nonlinear Temkin model. The maximum sorption capacities of Sr2+ by PA, PS and PP were 31.8, 51.4 and 52.4 μg g-1, respectively, with the initial Sr2+concentration of 3400 μg L-1. The phenomenological models adequately described the sorption kinetics data, concluding that the internal diffusion was the limiting step for Sr2+ sorption onto PS; while the external and internal diffusion were the slowest steps in the case of PA and PP. The MD study revealed that the main sorption mechanism was electrostatic interaction. The interaction energies of PA-SrCl2, PS-SrCl2, and PP-SrCl2 were -5.638, -6.418, and -13.05 kcal mol-1.
中文翻译:

Sr2 +吸附到微塑料上的平衡,动力学和分子动力学模型。
在全球环境中,微塑料(MPs)成为普遍存在的污染物,它可能会吸收水生环境中的金属离子,从而造成不利的后果。Sr 2+和MP之间的相互作用以及所涉及的机制尚未研究。在这里,我们研究了聚酰胺(PA),聚苯乙烯(PS)和聚丙烯(PP)对Sr 2+的吸附行为。建立了三种现象学数学模型,并将其应用于描述吸附过程中的限速步骤。还进行了分子动力学(MD)模拟以研究吸附机理。结果表明,非线性Temkin模型给出了最佳等温线。Sr 2+的最大吸附容量通过PA,PS和PP测得的Sr 2+初始浓度分别为31.8、51.4和52.4μgg -1,L -1。现象学模型充分描述了吸附动力学数据,认为内部扩散是Sr 2+吸附到PS上的限制步骤。而在PA和PP情况下,内部和内部扩散是最慢的步骤。MD研究表明,主要的吸附机理是静电相互作用。PA-SrCl 2,PS-SrCl 2和PP-SrCl 2的相互作用能为-5.638,-6.418和-13.05 kcal mol -1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号