European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-06-26 , DOI: 10.1016/j.ejmech.2020.112507 Gangqiang Yang 1 , Meng Gao 1 , Yixiao Sun 1 , Conghui Wang 1 , Xiaojuan Fang 1 , Hongyan Gao 1 , Wenshuang Diao 1 , Hui Yu 2
|
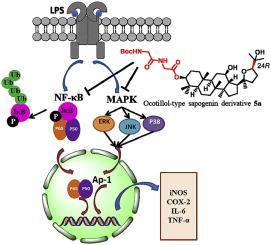
Ocotillol-type sapogenins (OTS) are major ginsenoside metabolites in human hepatic tissue. In order to better utilize OTS and derivatives thereof as anti-inflammatory compounds, present study produced multiple novel 3-amino acid OTS derivatives and evaluated their anti-inflammatory activity in vitro. The nitric oxide (NO) inhibitory activity of these compounds was used for OTS structure-activity relationship (SAR) evaluations, revealing that both R/S stereochemistry at C-24 and the amino acid type at C-3 influence such NO inhibitory activity. This activity was highest for an N-Boc-protected neutral aliphatic amino acid derivative of 24R-OTS (5a), which performed better than even hydrocortisone sodium succinate in vitro. Compound 5a was also able to markedly suppress the LPS-induced upregulation of TNF-α, IL-6, iNOS, and COX-2 via the NF-κB and MAPK pathways. This suggests that OTS derivatives may be valuable anti-inflammatory compounds worthy of further preclinical evaluation.
中文翻译:

Ocotillol型皂苷元的3个氨基酸衍生物的设计,合成和抗炎活性。
Ocotillol型皂甙元(OTS)是人肝组织中主要的人参皂甙代谢产物。为了更好地利用OTS及其衍生物作为抗炎化合物,本研究产生了多种新颖的3-氨基酸OTS衍生物,并评价了它们的体外抗炎活性。这些化合物的一氧化氮(NO)抑制活性用于OTS构效关系(SAR)评估,揭示了C-24的R / S立体化学和C-3的氨基酸类型均会影响这种NO抑制活性。对于24 R -OTS的N - Boc保护的中性脂肪族氨基酸衍生物,该活性最高(5a),其体外效果甚至优于氢化可的松琥珀酸钠。化合物5a还能够通过NF-κB和MAPK途径显着抑制LPS诱导的TNF-α,IL-6,iNOS和COX-2的上调。这表明OTS衍生物可能是有价值的抗炎化合物,值得进一步的临床前评估。



























 京公网安备 11010802027423号
京公网安备 11010802027423号