当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural changes in the model of the outer cell membrane of Gram-negative bacteria interacting with melittin: an in situ spectroelectrochemical study
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-06-24 , DOI: 10.1039/d0fd00039f Izabella Brand 1 , Bishoy Khairalla 1
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-06-24 , DOI: 10.1039/d0fd00039f Izabella Brand 1 , Bishoy Khairalla 1
Affiliation
|
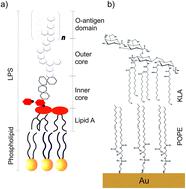
The cell membrane of Gram-negative bacteria interacting with an antimicrobial peptide presents a complex supramolecular assembly. Fabrication of models of bacterial cell membranes remains a large experimental challenge. Langmuir–Blodgett and Langmuir–Schaefer (LS–LB) transfer makes possible the deposition of multicomponent asymmetric lipid bilayers onto a gold surface. Two lipids: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) and di[3-deoxy-D-manno-octulosonyl]-lipid A (KLA) were used to deposit a model of the outer membrane of Gram-negative bacteria on the Au(111) substrate. The use of gold as the solid substrate enables control of the membrane potential. Molecular scale changes in the model membrane exposed to physiological electric fields and interacting with melittin antimicrobial peptide are discussed in this paper. The interaction of the outer membrane with melittin leads to an increase in the membrane capacitance and permeability to ions and water. The stability of the outer membrane with bound melittin decreases at positive membrane potentials. In situ polarization modulation infrared reflection absorption spectroscopy is used to investigate membrane potential-dependent changes in the structure of the outer membrane interacting with melittin. The hydration of the ester carbonyl groups is not affected by the interaction with melittin. However, the orientation and hydrogen bond network with the carboxylate groups in KLA changes drastically after POPE–KLA bilayer interacts with melittin. We propose that the positively charged groups in the amino acids present at the C-terminus of the peptide interact directly with the polar head group of KLA. Simultaneously, the packing order in hydrocarbon chains in the membrane with bound melittin increases. A hydrophobic match between the chains in the lipids and the peptide, which spans the membrane, seems to be responsible for the ordering of the hydrocarbon chains region of the bilayer. The N-terminus enters into the hydrophobic region of the membrane and forms a channel to the hydrophilic head groups in POPE.
中文翻译:

革兰氏阴性菌外细胞膜模型与蜂毒肽相互作用的结构变化:原位光谱电化学研究
与抗菌肽相互作用的革兰氏阴性菌的细胞膜呈现出复杂的超分子组装体。细菌细胞膜模型的制造仍然是一个巨大的实验挑战。Langmuir-Blodgett 和 Langmuir-Schaefer (LS-LB) 转移使多组分不对称脂质双层沉积在金表面上成为可能。两种脂质:1-palmitoyl-2- oleoyl- sn-glycero -3-phosphoethanolamine ( POPE ) 和二 [3- deoxy- D -manno-octulosonyl]-lipid A ( KLA) 用于在 Au(111) 底物上沉积革兰氏阴性菌外膜模型。使用金作为固体基质可以控制膜电位。本文讨论了暴露于生理电场并与蜂毒肽抗菌肽相互作用的模型膜的分子尺度变化。外膜与蜂毒肽的相互作用导致膜电容和对离子和水的渗透性增加。具有结合蜂毒肽的外膜的稳定性在膜电位为正时降低。原位偏振调制红外反射吸收光谱用于研究与蜂毒蛋白相互作用的外膜结构的膜电位依赖性变化。酯羰基的水合不受与蜂毒肽相互作用的影响。然而,在POPE - KLA双层与蜂毒肽相互作用后, KLA中羧酸酯基团的方向和氢键网络发生了巨大变化。我们建议存在于肽 C 末端的氨基酸中带正电荷的基团直接与KLA的极性头部基团相互作用. 同时,结合蜂毒肽的膜中烃链的堆积顺序增加。脂质中的链和跨越膜的肽之间的疏水匹配似乎是双层烃链区域排序的原因。N-末端进入膜的疏水区域并形成通向POPE中亲水头部基团的通道。
更新日期:2020-06-24
中文翻译:

革兰氏阴性菌外细胞膜模型与蜂毒肽相互作用的结构变化:原位光谱电化学研究
与抗菌肽相互作用的革兰氏阴性菌的细胞膜呈现出复杂的超分子组装体。细菌细胞膜模型的制造仍然是一个巨大的实验挑战。Langmuir-Blodgett 和 Langmuir-Schaefer (LS-LB) 转移使多组分不对称脂质双层沉积在金表面上成为可能。两种脂质:1-palmitoyl-2- oleoyl- sn-glycero -3-phosphoethanolamine ( POPE ) 和二 [3- deoxy- D -manno-octulosonyl]-lipid A ( KLA) 用于在 Au(111) 底物上沉积革兰氏阴性菌外膜模型。使用金作为固体基质可以控制膜电位。本文讨论了暴露于生理电场并与蜂毒肽抗菌肽相互作用的模型膜的分子尺度变化。外膜与蜂毒肽的相互作用导致膜电容和对离子和水的渗透性增加。具有结合蜂毒肽的外膜的稳定性在膜电位为正时降低。原位偏振调制红外反射吸收光谱用于研究与蜂毒蛋白相互作用的外膜结构的膜电位依赖性变化。酯羰基的水合不受与蜂毒肽相互作用的影响。然而,在POPE - KLA双层与蜂毒肽相互作用后, KLA中羧酸酯基团的方向和氢键网络发生了巨大变化。我们建议存在于肽 C 末端的氨基酸中带正电荷的基团直接与KLA的极性头部基团相互作用. 同时,结合蜂毒肽的膜中烃链的堆积顺序增加。脂质中的链和跨越膜的肽之间的疏水匹配似乎是双层烃链区域排序的原因。N-末端进入膜的疏水区域并形成通向POPE中亲水头部基团的通道。











































 京公网安备 11010802027423号
京公网安备 11010802027423号