当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural determinants of macrocyclization in substrate-controlled lanthipeptide biosynthetic pathways
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-25 , DOI: 10.1039/d0sc01651a Silvia C. Bobeica 1, 2, 3, 4 , Lingyang Zhu 2, 3, 4, 5 , Jeella Z. Acedo 1, 2, 3, 4 , Weixin Tang 1, 2, 3, 4 , Wilfred A. van der Donk 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-25 , DOI: 10.1039/d0sc01651a Silvia C. Bobeica 1, 2, 3, 4 , Lingyang Zhu 2, 3, 4, 5 , Jeella Z. Acedo 1, 2, 3, 4 , Weixin Tang 1, 2, 3, 4 , Wilfred A. van der Donk 1, 2, 3, 4
Affiliation
|
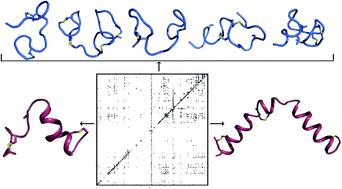
Lanthipeptides are characterized by thioether crosslinks formed by post-translational modifications. The cyclization process that favors a single ring pattern over many other possible ring patterns has been the topic of much speculation. Recent studies suggest that for some systems the cyclization pattern and stereochemistry is determined not by the enzyme, but by the sequence of the precursor peptide. However, the factors that govern the outcome of the cyclization process are not understood. This study presents the three-dimensional structures of seven lanthipeptides determined by nuclear magnetic resonance spectroscopy, including five prochlorosins and the two peptides that make up cytolysin, a virulence factor produced by Enterococcus faecalis that is directly linked to human disease. These peptides were chosen because their substrate sequence determines either the ring pattern (prochlorosins) or the stereochemistry of cyclization (cytolysins). We present the structures of prochlorosins 1.1, 2.1, 2.8, 2.10 and 2.11, the first three-dimensional structures of prochlorosins. Our findings provide insights into the molecular determinants of cyclization as well as why some prochlorosins may be better starting points for library generation than others. The structures of the large and small subunits of the enterococcal cytolysin show that these peptides have long helical stretches, a rare observation for lanthipeptides characterized to date. These helices may explain their pore forming activity and suggest that the small subunit may recognize a molecular target followed by recruitment of the large subunit to span the membrane.
中文翻译:

底物控制的多肽多肽生物合成途径中大环化的结构决定因素。
多肽肽的特征在于通过翻译后修饰形成的硫醚交联。比许多其他可能的环形图案更偏爱单个环形图案的环化过程已成为许多猜测的主题。最近的研究表明,对于某些系统,环化模式和立体化学不是由酶决定的,而是由前体肽的序列决定的。但是,尚不了解控制环化过程结果的因素。这项研究提出了由核磁共振波谱确定的七个七肽的三维结构,包括五个原绿素和构成溶血素的两个肽,这是屎肠球菌产生的一种致病因子。与人类疾病直接相关。选择这些肽是因为它们的底物序列决定了环型(原绿素)或环化的立体化学(溶细胞素)。我们介绍了原花青素的结构1.1、2.1、2.8、2.10和2.11,这是原花青素的第一个三维结构。我们的发现提供了关于环化的分子决定因素的见解,以及为什么某些原绿素可能比其他一些更容易成为文库生成的起点。肠球菌溶血素的大亚基和小亚基的结构表明,这些肽具有长的螺旋形延伸,这是迄今为止尚未鉴定的瘦肽的观察结果。
更新日期:2020-07-01
中文翻译:

底物控制的多肽多肽生物合成途径中大环化的结构决定因素。
多肽肽的特征在于通过翻译后修饰形成的硫醚交联。比许多其他可能的环形图案更偏爱单个环形图案的环化过程已成为许多猜测的主题。最近的研究表明,对于某些系统,环化模式和立体化学不是由酶决定的,而是由前体肽的序列决定的。但是,尚不了解控制环化过程结果的因素。这项研究提出了由核磁共振波谱确定的七个七肽的三维结构,包括五个原绿素和构成溶血素的两个肽,这是屎肠球菌产生的一种致病因子。与人类疾病直接相关。选择这些肽是因为它们的底物序列决定了环型(原绿素)或环化的立体化学(溶细胞素)。我们介绍了原花青素的结构1.1、2.1、2.8、2.10和2.11,这是原花青素的第一个三维结构。我们的发现提供了关于环化的分子决定因素的见解,以及为什么某些原绿素可能比其他一些更容易成为文库生成的起点。肠球菌溶血素的大亚基和小亚基的结构表明,这些肽具有长的螺旋形延伸,这是迄今为止尚未鉴定的瘦肽的观察结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号