当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical condensation between benzylic alcohols and acetamides to form 3-arylpropanamides
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-25 , DOI: 10.1039/d0sc02948c Kobra Azizi 1, 2, 3 , Robert Madsen 1, 2, 3
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-25 , DOI: 10.1039/d0sc02948c Kobra Azizi 1, 2, 3 , Robert Madsen 1, 2, 3
Affiliation
|
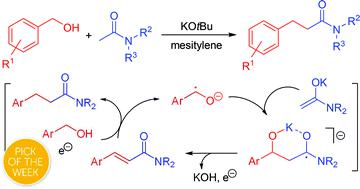
A new radical condensation reaction is developed where benzylic alcohols and acetamides are coupled to generate 3-arylpropanamides with water as the only byproduct. The transformation is performed with potassium tert-butoxide as the only additive and gives rise to a variety of 3-arylpropanamides in good yields. The mechanism has been investigated experimentally with labelled substrates, trapping experiments and spectroscopic measurements. The findings indicate a radical pathway where potassium tert-butoxide is believed to serve a dual role as both base and radical initiator. The radical anion of the benzylic alcohol is proposed as the key intermediate, which undergoes coupling with the enolate of the amide to form the new C–C bond. Subsequent elimination to the corresponding cinnamamide and olefin reduction then affords the 3-arylpropanamides.
中文翻译:

苄醇与乙酰胺之间的自由基缩合形成3-芳基丙酰胺
开发了一种新的自由基缩合反应,其中将苄醇和乙酰胺偶联以生成3-芳基丙酰胺,而水是唯一的副产物。用叔丁醇钾作为唯一添加剂进行转化,并以高收率产生各种3-芳基丙酰胺。已通过标记底物,捕获实验和光谱测量对这种机理进行了实验研究。研究结果表明自由基途径,其中钾叔据信-丁醇盐起碱和自由基引发剂的双重作用。提出了苄醇的自由基阴离子作为关键中间体,该中间体与酰胺的烯酸酯进行偶联形成新的C–C键。随后消除为相应的肉桂酰胺,然后烯烃还原得到3-芳基丙酰胺。
更新日期:2020-08-05
中文翻译:

苄醇与乙酰胺之间的自由基缩合形成3-芳基丙酰胺
开发了一种新的自由基缩合反应,其中将苄醇和乙酰胺偶联以生成3-芳基丙酰胺,而水是唯一的副产物。用叔丁醇钾作为唯一添加剂进行转化,并以高收率产生各种3-芳基丙酰胺。已通过标记底物,捕获实验和光谱测量对这种机理进行了实验研究。研究结果表明自由基途径,其中钾叔据信-丁醇盐起碱和自由基引发剂的双重作用。提出了苄醇的自由基阴离子作为关键中间体,该中间体与酰胺的烯酸酯进行偶联形成新的C–C键。随后消除为相应的肉桂酰胺,然后烯烃还原得到3-芳基丙酰胺。









































 京公网安备 11010802027423号
京公网安备 11010802027423号