当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ K-edge X-ray absorption spectroscopy of the ligand environment of single-site Au/C catalysts during acetylene hydrochlorination
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-24 , DOI: 10.1039/d0sc02152k Grazia Malta 1, 2, 3, 4, 5 , Simon A. Kondrat 5, 6, 7, 8 , Simon J. Freakley 5, 6, 9, 10 , David J. Morgan 1, 2, 3, 4, 5 , Emma K. Gibson 2, 5, 11, 12, 13 , Peter P. Wells 2, 5, 14, 15, 16 , Matteo Aramini 5, 16, 17, 18 , Diego Gianolio 5, 16, 17, 18 , Paul B. J. Thompson 19, 20, 21, 22, 23 , Peter Johnston 5, 24, 25, 26 , Graham J. Hutchings 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-24 , DOI: 10.1039/d0sc02152k Grazia Malta 1, 2, 3, 4, 5 , Simon A. Kondrat 5, 6, 7, 8 , Simon J. Freakley 5, 6, 9, 10 , David J. Morgan 1, 2, 3, 4, 5 , Emma K. Gibson 2, 5, 11, 12, 13 , Peter P. Wells 2, 5, 14, 15, 16 , Matteo Aramini 5, 16, 17, 18 , Diego Gianolio 5, 16, 17, 18 , Paul B. J. Thompson 19, 20, 21, 22, 23 , Peter Johnston 5, 24, 25, 26 , Graham J. Hutchings 1, 2, 3, 4, 5
Affiliation
|
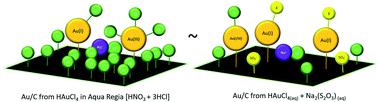
The replacement of HgCl2/C with Au/C as a catalyst for acetylene hydrochlorination represents a significant reduction in the environmental impact of this industrial process. Under reaction conditions atomically dispersed cationic Au species are the catalytic active site, representing a large-scale application of heterogeneous single-site catalysts. While the metal nuclearity and oxidation state under operating conditions has been investigated in catalysts prepared from aqua regia and thiosulphate, limited studies have focused on the ligand environment surrounding the metal centre. We now report K-edge soft X-ray absorption spectroscopy of the Cl and S ligand species used to stabilise these isolated cationic Au centres in the harsh reaction conditions. We demonstrate the presence of three distinct Cl species in the materials; inorganic Cl−, Au–Cl, and C–Cl and how these species evolve during reaction. Direct evidence of Au–S interactions is confirmed in catalysts prepared using thiosulfate precursors which show high stability towards reduction to inactive metal nanoparticles. This stability was clear during gas switching experiments, where exposure to C2H2 alone did not dramatically alter the Au electronic structure and consequently did not deactivate the thiosulfate catalyst.
中文翻译:

乙炔盐酸化过程中单中心Au / C催化剂配体环境的原位K边缘X射线吸收光谱
用Au / C代替HgCl 2 / C作为乙炔盐酸盐化的催化剂代表该工业过程对环境的影响显着降低。在反应条件下,原子分散的阳离子Au物种是催化活性中心,代表了非均相单中心催化剂的大规模应用。尽管在王水制备的催化剂中研究了操作条件下的金属核化和氧化态和硫代硫酸盐,有限的研究集中在金属中心周围的配体环境上。我们现在报告用于在苛刻的反应条件下稳定这些孤立的阳离子Au中心的Cl和S配体物种的K边缘软X射线吸收光谱。我们证明了材料中存在三种不同的Cl物种;无机氯-,AU-Cl和C-Cl和如何反应期间这些物种进化。使用硫代硫酸盐前体制备的催化剂证实了Au-S相互作用的直接证据,该催化剂对还原成惰性金属纳米粒子表现出很高的稳定性。在暴露于C 2 H 2的气体转换实验中,这种稳定性很明显 单独使用不会显着改变Au电子结构,因此不会使硫代硫酸盐催化剂失活。
更新日期:2020-07-15
中文翻译:

乙炔盐酸化过程中单中心Au / C催化剂配体环境的原位K边缘X射线吸收光谱
用Au / C代替HgCl 2 / C作为乙炔盐酸盐化的催化剂代表该工业过程对环境的影响显着降低。在反应条件下,原子分散的阳离子Au物种是催化活性中心,代表了非均相单中心催化剂的大规模应用。尽管在王水制备的催化剂中研究了操作条件下的金属核化和氧化态和硫代硫酸盐,有限的研究集中在金属中心周围的配体环境上。我们现在报告用于在苛刻的反应条件下稳定这些孤立的阳离子Au中心的Cl和S配体物种的K边缘软X射线吸收光谱。我们证明了材料中存在三种不同的Cl物种;无机氯-,AU-Cl和C-Cl和如何反应期间这些物种进化。使用硫代硫酸盐前体制备的催化剂证实了Au-S相互作用的直接证据,该催化剂对还原成惰性金属纳米粒子表现出很高的稳定性。在暴露于C 2 H 2的气体转换实验中,这种稳定性很明显 单独使用不会显着改变Au电子结构,因此不会使硫代硫酸盐催化剂失活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号