当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Late-stage and strain-accelerated oxidation enabled synthesis of haouamine A
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-24 , DOI: 10.1039/d0sc02299c Kun Ho Kenny Park 1 , Antonio Rizzo 1 , David Y-K Chen 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-24 , DOI: 10.1039/d0sc02299c Kun Ho Kenny Park 1 , Antonio Rizzo 1 , David Y-K Chen 1
Affiliation
|
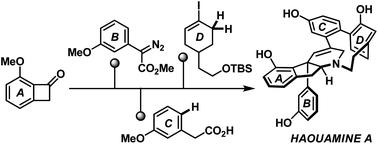
Herein we report a new synthetic entry to the strained cyclophane alkaloid natural product, haouamine A. The successful strategy featured a rhodium-catalyzed diazo-insertion reaction to install the all-carbon quaternary center and a rhodium-catalyzed intramolecular aziridination reaction to establish the nitrogen-bearing stereocenter, of the target molecule. Most notably, a late-stage, site-selective and strain-accelerated oxidation of a “deoxygenated” macrocyclic intermediate was successfully implemented, and in doing so provided a novel solution to the infamous biphenol cyclophane system of haouamine A.
中文翻译:

后期和应变加速氧化促进了 haouamine A 的合成
在此,我们报告了一种新的合成方法,用于过滤环芳生物碱天然产物 haouamine A。该成功的策略包括铑催化的重氮插入反应以安装全碳四元中心和铑催化的分子内氮丙啶化反应以建立氮-目标分子的立体中心。最值得注意的是,成功地实现了“脱氧”大环中间体的后期、位点选择性和应变加速氧化,从而为臭名昭著的 haouamine A 联苯酚环芳系统提供了一种新颖的解决方案。
更新日期:2020-08-12
中文翻译:

后期和应变加速氧化促进了 haouamine A 的合成
在此,我们报告了一种新的合成方法,用于过滤环芳生物碱天然产物 haouamine A。该成功的策略包括铑催化的重氮插入反应以安装全碳四元中心和铑催化的分子内氮丙啶化反应以建立氮-目标分子的立体中心。最值得注意的是,成功地实现了“脱氧”大环中间体的后期、位点选择性和应变加速氧化,从而为臭名昭著的 haouamine A 联苯酚环芳系统提供了一种新颖的解决方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号