当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyrazolone ligation-mediated versatile sequential bioconjugations
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-24 , DOI: 10.1039/d0sc02466j Melrose Mailig 1, 2, 3 , Fa Liu 1, 2, 3
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-24 , DOI: 10.1039/d0sc02466j Melrose Mailig 1, 2, 3 , Fa Liu 1, 2, 3
Affiliation
|
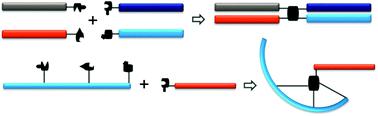
Bioconjugation chemistries are critical tools in biotherapeutics discovery. The past efforts have been exclusively focused on two-segment conjugations. However, emerging research directions, such as polypharmacy biotherapeutics, desire multiple-component bioconjugations where more than two pharmacologically related biomolecules can be assembled into a single construct in high efficiency. We present here a set of sequential bioconjugation chemistries centered on a pyrazolone structural motif. It starts with a clickable “pyrazolone ligation” between a hydrazine group and a β-ketoester moiety followed by the conjugation between the newly formed pyrazolone core and an aldehyde-bearing biomolecule through a Knoevenagel reaction forming a Michael addition acceptor that can effectively capture a thiol-bearing biomolecule. When utilized intermolecularly, it quickly assembles four segments together forming a quadruple functional construct. When applied intramolecularly, it offers a set of highly diverse biomolecule scaffolds including stapled peptides and poly-macrocyclic peptides. We envision broad utilities of such sequential ligation chemistries.
中文翻译:

吡唑啉酮连接介导的多功能顺序生物偶联
生物缀合化学是生物治疗学发现中的关键工具。过去的努力仅集中在两段式共轭上。然而,新兴的研究方向,例如多药房生物治疗学,要求多组分生物缀合,其中可以将两个以上药理学相关的生物分子高效地组装成单个构建体。我们在这里提出了一套以吡唑啉酮结构基序为中心的顺序生物缀合化学。它以肼基与β-酮酸酯部分之间的可点击的“吡唑啉酮连接”开始,然后通过新形成的吡唑酮核与含醛的生物分子通过形成Knoevenagel反应的共轭反应形成可有效捕获硫醇的迈克尔加成受体,开始轴承生物分子。当用于分子间时 它迅速将四个段组装在一起,形成一个四重功能结构。当在分子内应用时,它提供了一组高度多样化的生物分子支架,包括固定肽和聚大环肽。我们设想了这种顺序连接化学的广泛用途。
更新日期:2020-07-15
中文翻译:

吡唑啉酮连接介导的多功能顺序生物偶联
生物缀合化学是生物治疗学发现中的关键工具。过去的努力仅集中在两段式共轭上。然而,新兴的研究方向,例如多药房生物治疗学,要求多组分生物缀合,其中可以将两个以上药理学相关的生物分子高效地组装成单个构建体。我们在这里提出了一套以吡唑啉酮结构基序为中心的顺序生物缀合化学。它以肼基与β-酮酸酯部分之间的可点击的“吡唑啉酮连接”开始,然后通过新形成的吡唑酮核与含醛的生物分子通过形成Knoevenagel反应的共轭反应形成可有效捕获硫醇的迈克尔加成受体,开始轴承生物分子。当用于分子间时 它迅速将四个段组装在一起,形成一个四重功能结构。当在分子内应用时,它提供了一组高度多样化的生物分子支架,包括固定肽和聚大环肽。我们设想了这种顺序连接化学的广泛用途。











































 京公网安备 11010802027423号
京公网安备 11010802027423号