当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Enantioselective Michael Addition between 3-(3-hydroxy-1H-pyrazol-1-yl)Oxindole and β-Nitrostyrene for the Preparation of Chiral Disubstituted Oxindoles.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-25 , DOI: 10.1021/acs.joc.9b03337 Xiang-Jia Song 1, 2 , Hong-Xia Ren 1, 2 , Min Xiang 1, 2 , Chen-Yi Li 1, 2 , Ying Zou 1, 2 , Xia Li 1, 2 , Zhi-Cheng Huang 1, 2 , Fang Tian 1 , Li-Xin Wang 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-25 , DOI: 10.1021/acs.joc.9b03337 Xiang-Jia Song 1, 2 , Hong-Xia Ren 1, 2 , Min Xiang 1, 2 , Chen-Yi Li 1, 2 , Ying Zou 1, 2 , Xia Li 1, 2 , Zhi-Cheng Huang 1, 2 , Fang Tian 1 , Li-Xin Wang 1
Affiliation
|
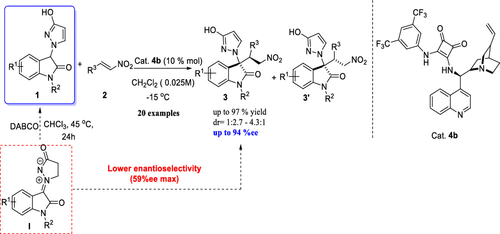
A new enantioselective Michael addition between 3-(3-hydroxy-1H-pyrazol-1-yl)oxindole, a new synthon generated from isatin N,N′-cyclic azomethine imine 1,3-dipole, and β-nitrostyrene has been disclosed. A series of chiral 3-(3-oxo-2,3-dihydro-1H-pyrazol-1-yl) disubstituted oxindoles were obtained in excellent results (up to 97% yield, up to 94% ee) with moderate to good diastereoselectivities (up to 4.3:1 dr).
中文翻译:

3-(3-羟基-1H-吡唑-1-基)O和β-硝基苯乙烯之间的有机催化对映选择性迈克尔加成反应,用于制备手性二取代的吲哚。
在3-(3-羟基-1 H-吡唑-1-基)恶吲哚之间的一种新的对映选择性迈克尔加成反应,它是由Isatin N,N'-环偶氮甲亚胺亚胺1,3-偶极和β-硝基苯乙烯生成的新合成子。披露。获得了一系列优异的收率(最高97%,最高94%ee)的手性3-(3-oxo-2,3-dihydro-1 H -pyrazol-1-yl)双取代的吲哚。非对映选择性(达4.3:1 dr)。
更新日期:2020-07-17
中文翻译:

3-(3-羟基-1H-吡唑-1-基)O和β-硝基苯乙烯之间的有机催化对映选择性迈克尔加成反应,用于制备手性二取代的吲哚。
在3-(3-羟基-1 H-吡唑-1-基)恶吲哚之间的一种新的对映选择性迈克尔加成反应,它是由Isatin N,N'-环偶氮甲亚胺亚胺1,3-偶极和β-硝基苯乙烯生成的新合成子。披露。获得了一系列优异的收率(最高97%,最高94%ee)的手性3-(3-oxo-2,3-dihydro-1 H -pyrazol-1-yl)双取代的吲哚。非对映选择性(达4.3:1 dr)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号