当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Catalysis Upon Helically Chiral Loratadine Analogs Unveils Enantiomer-Dependent Antihistamine Activity
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-24 , DOI: 10.1021/jacs.0c03904 Elizabeth A Stone 1 , Kara J Cutrona 1 , Scott J Miller 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-24 , DOI: 10.1021/jacs.0c03904 Elizabeth A Stone 1 , Kara J Cutrona 1 , Scott J Miller 1
Affiliation
|
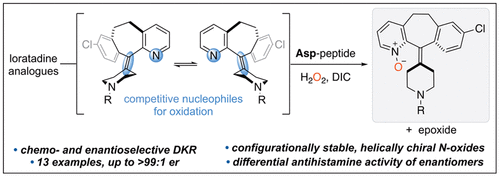
Analogs of the conformationally dynamic Claritin® (loratadine) and Clarinex® (desloratadine) scaffolds have been enantio- and chemoselectively N-oxidized using an aspartic acid containing peptide catalyst to afford stable, helically chiral products in up to >99:1 er. The conformational dynamics and enantiomeric stability of the N-oxide products have been investigated experimentally and computationally with the aid of crystallographic data. Furthermore, biological assays show that rigidifying the core structure of loratadine and related analogs through N-oxidation affects antihistamine activity in an enantiomer-dependent fashion. Computational docking studies illustrate the observed activity differences.
中文翻译:

螺旋手性氯雷他定类似物的不对称催化揭示了对映异构体依赖的抗组胺活性
构象动态 Claritin®(氯雷他定)和 Clarinex®(地氯雷他定)支架的类似物已使用含天冬氨酸的肽催化剂对映体和化学选择性 N-氧化,以提供高达 >99:1 er 的稳定螺旋手性产品。N-氧化物产物的构象动力学和对映体稳定性已经在晶体学数据的帮助下通过实验和计算进行了研究。此外,生物测定表明,通过 N-氧化使氯雷他定和相关类似物的核心结构刚性化会以对映异构体依赖的方式影响抗组胺药活性。计算对接研究说明了观察到的活性差异。
更新日期:2020-06-24
中文翻译:

螺旋手性氯雷他定类似物的不对称催化揭示了对映异构体依赖的抗组胺活性
构象动态 Claritin®(氯雷他定)和 Clarinex®(地氯雷他定)支架的类似物已使用含天冬氨酸的肽催化剂对映体和化学选择性 N-氧化,以提供高达 >99:1 er 的稳定螺旋手性产品。N-氧化物产物的构象动力学和对映体稳定性已经在晶体学数据的帮助下通过实验和计算进行了研究。此外,生物测定表明,通过 N-氧化使氯雷他定和相关类似物的核心结构刚性化会以对映异构体依赖的方式影响抗组胺药活性。计算对接研究说明了观察到的活性差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号