当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Regeneration of Spent Alkaline Absorbent from Direct Air Capture.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-06-25 , DOI: 10.1021/acs.est.0c01977 Qingdian Shu 1, 2 , Louis Legrand 1, 2 , Philipp Kuntke 1, 2 , Michele Tedesco 1 , Hubertus V M Hamelers 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-06-25 , DOI: 10.1021/acs.est.0c01977 Qingdian Shu 1, 2 , Louis Legrand 1, 2 , Philipp Kuntke 1, 2 , Michele Tedesco 1 , Hubertus V M Hamelers 1
Affiliation
|
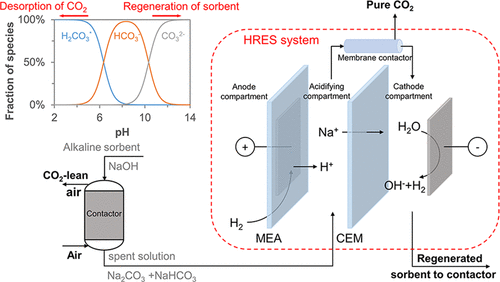
CO2 capture from the atmosphere (or direct air capture) is widely recognized as a promising solution to reach negative emissions, and technologies using alkaline solutions as absorbent have already been demonstrated on a full scale. In the conventional temperature swing process, the subsequent regeneration of the alkaline solution is highly energy-demanding. In this study, we experimentally demonstrate simultaneous solvent regeneration and CO2 desorption in a continuous system using a H2-recycling electrochemical cell. A pH gradient is created in the electrochemical cell so that CO2 is desorbed at a low pH, while an alkaline capture solution (NaOH) is regenerated at high pH. By testing the cell under different working conditions, we experimentally achieved CO2 desorption with an energy consumption of 374 kJ·mol–1 CO2 and a CO2 purity higher than 95%. Moreover, our theoretical calculations show that a minimum energy consumption of 164 kJ·mol–1 CO2 could be achieved. Overall, the H2-recycling electrochemical cell allowed us to accomplish the simultaneous desorption of high-purity CO2 stream and regeneration of up to 59% of the CO2 capture capacity of the absorbent. These results are promising toward the upscaling of an energy-effective process for direct air capture.
中文翻译:

直接空气捕获中废碱性吸收剂的电化学再生。
从大气中捕获CO 2 (或直接空气捕获)被广泛认为是实现负排放的有前途的解决方案,并且使用碱性溶液作为吸收剂的技术已经得到全面论证。在传统的变温过程中,碱性溶液的后续再生非常耗能。在本研究中,我们通过实验证明了使用 H 2循环电化学电池在连续系统中同时进行溶剂再生和 CO 2解吸。在电化学电池中产生pH梯度,使得CO 2在低pH值下解吸,而碱性捕获溶液(NaOH)在高pH值下再生。通过不同工况下的测试,我们实验性地实现了CO 2解吸,能耗为374 kJ·mol –1 CO 2 ,CO 2纯度高于95%。此外,我们的理论计算表明,可以实现164 kJ·mol –1 CO 2的最低能耗。总体而言,H 2循环电化学电池使我们能够同时完成高纯度CO 2流的解吸和吸收剂高达59%的CO 2捕获能力的再生。这些结果有望提高直接空气捕获的节能过程的规模。
更新日期:2020-07-21
中文翻译:

直接空气捕获中废碱性吸收剂的电化学再生。
从大气中捕获CO 2 (或直接空气捕获)被广泛认为是实现负排放的有前途的解决方案,并且使用碱性溶液作为吸收剂的技术已经得到全面论证。在传统的变温过程中,碱性溶液的后续再生非常耗能。在本研究中,我们通过实验证明了使用 H 2循环电化学电池在连续系统中同时进行溶剂再生和 CO 2解吸。在电化学电池中产生pH梯度,使得CO 2在低pH值下解吸,而碱性捕获溶液(NaOH)在高pH值下再生。通过不同工况下的测试,我们实验性地实现了CO 2解吸,能耗为374 kJ·mol –1 CO 2 ,CO 2纯度高于95%。此外,我们的理论计算表明,可以实现164 kJ·mol –1 CO 2的最低能耗。总体而言,H 2循环电化学电池使我们能够同时完成高纯度CO 2流的解吸和吸收剂高达59%的CO 2捕获能力的再生。这些结果有望提高直接空气捕获的节能过程的规模。











































 京公网安备 11010802027423号
京公网安备 11010802027423号