当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cholesterol Constrains the Antigenic Configuration of the Membrane-Proximal Neutralizing HIV-1 Epitope.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-06-25 , DOI: 10.1021/acsinfecdis.0c00243 Johana Torralba 1, 2 , Igor de la Arada 1 , Pablo Carravilla 1, 2, 3, 4 , Sara Insausti 1, 2 , Edurne Rujas 1, 2 , Eneko Largo 1, 5 , Christian Eggeling 3, 4, 6 , José L R Arrondo 1, 2 , Beatriz Apellániz 1, 7 , José L Nieva 1, 2
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-06-25 , DOI: 10.1021/acsinfecdis.0c00243 Johana Torralba 1, 2 , Igor de la Arada 1 , Pablo Carravilla 1, 2, 3, 4 , Sara Insausti 1, 2 , Edurne Rujas 1, 2 , Eneko Largo 1, 5 , Christian Eggeling 3, 4, 6 , José L R Arrondo 1, 2 , Beatriz Apellániz 1, 7 , José L Nieva 1, 2
Affiliation
|
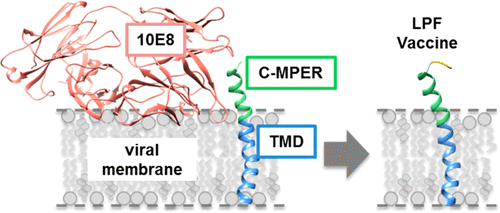
The envelope glycoprotein (Env) enables HIV-1 cell entry through fusion of host-cell and viral membranes induced by the transmembrane subunit gp41. Antibodies targeting the C-terminal sequence of the membrane-proximal external region (C-MPER) block the fusogenic activity of gp41 and achieve neutralization of divergent HIV-1 strains and isolates. Thus, recreating the structure that generates broadly neutralizing C-MPER antibodies during infection is a major goal in HIV vaccine development. Here, we have reconstituted a peptide termed CpreTM-TMD in a membrane environment. This peptide contains the C-MPER epitope and the minimum TMD residues required for the anchorage of the Env glycoprotein to the viral membrane. In addition, we have used antibody 10E8 variants to gauge the antigenic configuration attained by CpreTM-TMD as a function of the membrane cholesterol content, a functional determinant of the HIV envelope and liposome-based vaccines. Differential binding of the 10E8 variants and the trend of the IgG responses recovered from rabbits immunized with liposome-peptide formulations, suggested that cholesterol may restrict 10E8 accessibility to the C-MPER epitope. Our data ruled out the destabilization of the lipid bilayer architecture in CpreTM-TMD-containing membranes, and pointed to the perturbation of the helical conformation by lipid packing as the cause of the antigenic configuration loss induced by cholesterol. Overall, our results provide additional insights into the structural basis of the Env complex anchoring to membranes, and suggest new approaches to the design of effective immunogens directed against the near pan-neutralizing HIV-1 epitope C-MPER.
中文翻译:

胆固醇限制近膜中和 HIV-1 表位的抗原构型。
包膜糖蛋白 (Env) 通过跨膜亚基 gp41 诱导的宿主细胞和病毒膜融合,使 HIV-1 能够进入细胞。靶向近膜外部区域 C 端序列 (C-MPER) 的抗体可阻断 gp41 的融合活性,并实现不同 HIV-1 毒株和分离株的中和。因此,重建在感染过程中产生广泛中和 C-MPER 抗体的结构是 HIV 疫苗开发的一个主要目标。在这里,我们在膜环境中重建了一种名为 CpreTM-TMD 的肽。该肽含有 C-MPER 表位和将 Env 糖蛋白锚定到病毒膜所需的最小 TMD 残基。此外,我们还使用抗体 10E8 变体来测量 CpreTM-TMD 获得的抗原构型作为膜胆固醇含量的函数,膜胆固醇含量是 HIV 包膜和脂质体疫苗的功能决定因素。10E8 变体的差异结合和从用脂质体-肽制剂免疫的兔子中恢复的 IgG 反应趋势表明,胆固醇可能限制 10E8 对 C-MPER 表位的可及性。我们的数据排除了含有 CpreTM-TMD 的膜中脂质双层结构的不稳定,并指出脂质堆积对螺旋构象的扰动是胆固醇诱导的抗原构型丢失的原因。总的来说,我们的结果为锚定在膜上的 Env 复合物的结构基础提供了更多的见解,并提出了设计针对近乎泛中和的 HIV-1 表位 C-MPER 的有效免疫原的新方法。
更新日期:2020-08-14
中文翻译:

胆固醇限制近膜中和 HIV-1 表位的抗原构型。
包膜糖蛋白 (Env) 通过跨膜亚基 gp41 诱导的宿主细胞和病毒膜融合,使 HIV-1 能够进入细胞。靶向近膜外部区域 C 端序列 (C-MPER) 的抗体可阻断 gp41 的融合活性,并实现不同 HIV-1 毒株和分离株的中和。因此,重建在感染过程中产生广泛中和 C-MPER 抗体的结构是 HIV 疫苗开发的一个主要目标。在这里,我们在膜环境中重建了一种名为 CpreTM-TMD 的肽。该肽含有 C-MPER 表位和将 Env 糖蛋白锚定到病毒膜所需的最小 TMD 残基。此外,我们还使用抗体 10E8 变体来测量 CpreTM-TMD 获得的抗原构型作为膜胆固醇含量的函数,膜胆固醇含量是 HIV 包膜和脂质体疫苗的功能决定因素。10E8 变体的差异结合和从用脂质体-肽制剂免疫的兔子中恢复的 IgG 反应趋势表明,胆固醇可能限制 10E8 对 C-MPER 表位的可及性。我们的数据排除了含有 CpreTM-TMD 的膜中脂质双层结构的不稳定,并指出脂质堆积对螺旋构象的扰动是胆固醇诱导的抗原构型丢失的原因。总的来说,我们的结果为锚定在膜上的 Env 复合物的结构基础提供了更多的见解,并提出了设计针对近乎泛中和的 HIV-1 表位 C-MPER 的有效免疫原的新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号