当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Allylamine Derivatives via Intermolecular Aza‐Wacker‐Type Reaction Promoted by Palladium‐SPRIX Catalyst
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-24 , DOI: 10.1002/adsc.202000644 Abhijit Sen 1, 2 , Linpeng Zhu 1 , Shinobu Takizawa 1 , Kazuhiro Takenaka 1, 3 , Hiroaki Sasai 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-24 , DOI: 10.1002/adsc.202000644 Abhijit Sen 1, 2 , Linpeng Zhu 1 , Shinobu Takizawa 1 , Kazuhiro Takenaka 1, 3 , Hiroaki Sasai 1
Affiliation
|
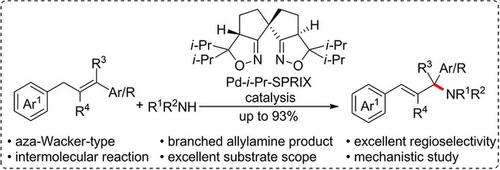
An intermolecular aza‐Wacker‐type reaction was developed. When a readily available olefin was treated with a nitrogen nucleophile in the presence of a Pd‐SPRIX complex and potassium persulfate, allylamine derivatives were obtained with high yield and excellent regioselectivity. The mechanistic studies showed that the reaction followed first‐order dependence on the olefin as well as palladium catalyst, but zero‐order dependence on the nitrogen nucleophile.
中文翻译:

钯-SPRIX催化剂促进的分子间Aza-Wacker型反应合成烯丙胺衍生物
发生了分子间的氮杂-瓦克型反应。当在Pd-SPRIX配合物和过硫酸钾存在下用氮亲核试剂处理易得的烯烃时,可以获得高收率和极高的区域选择性的烯丙胺衍生物。机理研究表明,该反应遵循对烯烃以及钯催化剂的一阶依赖性,但对氮亲核试剂的阶跃依赖性为零。
更新日期:2020-06-24
中文翻译:

钯-SPRIX催化剂促进的分子间Aza-Wacker型反应合成烯丙胺衍生物
发生了分子间的氮杂-瓦克型反应。当在Pd-SPRIX配合物和过硫酸钾存在下用氮亲核试剂处理易得的烯烃时,可以获得高收率和极高的区域选择性的烯丙胺衍生物。机理研究表明,该反应遵循对烯烃以及钯催化剂的一阶依赖性,但对氮亲核试剂的阶跃依赖性为零。









































 京公网安备 11010802027423号
京公网安备 11010802027423号