当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C3‐Thioester/‐Ester Substituted Linear Dienones: A Pluripotent Molecular Platform for Diversification via Cascade Pericyclic Reactions
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-24 , DOI: 10.1002/adsc.202000362 Abhijit Bankura 1 , Sandip Naskar 1 , Sabyasachi Roy Chowdhury 2 , Rajib Maity 1 , Sabyashachi Mishra 2 , Indrajit Das 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-24 , DOI: 10.1002/adsc.202000362 Abhijit Bankura 1 , Sandip Naskar 1 , Sabyasachi Roy Chowdhury 2 , Rajib Maity 1 , Sabyashachi Mishra 2 , Indrajit Das 1
Affiliation
|
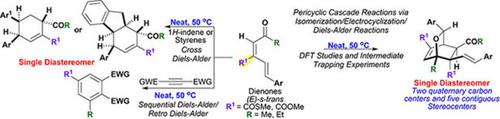
Substituted oxabicyclo derivatives bearing two quaternary carbon centers and five contiguous stereocenters have been synthesized from C3‐thioester/‐ester substituted dienones, a simple and linear pluripotent molecular platform. The conversion proceeds from neat reactants, possibly via a thermally‐driven pericyclic cascade manifold involving sequential (E)‐s‐trans to (E)‐s‐cis isomerization, oxa‐6π‐electrocyclization, and intermolecular, regioselective [4π+2π] cycloaddition. The proposed mechanism has been substantiated by intermediate trapping experiments and DFT studies. Such dienones have also been exploited to effect stereoselective cross Diels‐Alder cycloadditions with olefins and sequential Diels‐Alder/retro‐Diels‐Alder reactions with activated alkynes. The reaction is greatly influenced by the substituent effect exerted by the C3‐thioester/‐ester group.
中文翻译:

C 3-硫代酸酯/酯取代的线性二烯酮:通过级联周环反应实现多样化的多能分子平台
由一个简单且线性的多能分子平台C 3-硫酯/酯取代的二烯酮合成了具有两个季碳中心和五个连续立体中心的取代的氧杂双环衍生物。转化可从纯反应物开始,可能通过热驱动的周环级联歧管进行,涉及顺序(E)-s-反式向(E)-s-顺式异构化,oxa-6π-电环化和分子间,区域选择性[4π+2π]环加成反应。中间捕获实验和DFT研究证实了所提出的机制。此类二烯酮还被用于与烯烃进行立体选择性交叉Diels-Alder环加成反应,以及与活化炔烃的顺序Diels-Alder / retro-Diels-Alder反应。该反应在很大程度上受C 3-硫代酸酯/酯基所产生的取代作用的影响。
更新日期:2020-06-24
中文翻译:

C 3-硫代酸酯/酯取代的线性二烯酮:通过级联周环反应实现多样化的多能分子平台
由一个简单且线性的多能分子平台C 3-硫酯/酯取代的二烯酮合成了具有两个季碳中心和五个连续立体中心的取代的氧杂双环衍生物。转化可从纯反应物开始,可能通过热驱动的周环级联歧管进行,涉及顺序(E)-s-反式向(E)-s-顺式异构化,oxa-6π-电环化和分子间,区域选择性[4π+2π]环加成反应。中间捕获实验和DFT研究证实了所提出的机制。此类二烯酮还被用于与烯烃进行立体选择性交叉Diels-Alder环加成反应,以及与活化炔烃的顺序Diels-Alder / retro-Diels-Alder反应。该反应在很大程度上受C 3-硫代酸酯/酯基所产生的取代作用的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号