当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of isoniazid‐1,2,3‐triazole conjugates: Antitubercular, antimicrobial evaluation and molecular docking study
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-06-25 , DOI: 10.1002/jhet.4072 Adinath D. Badar 1 , Shubham M. Sulakhe 1 , Mahesh B. Muluk 1 , Naziya N. M. A. Rehman 2 , Prashant P. Dixit 2 , Prafulla B. Choudhari 3 , Estharla Madhu Rekha 4 , Dharmarajan Sriram 4 , Kishan P. Haval 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-06-25 , DOI: 10.1002/jhet.4072 Adinath D. Badar 1 , Shubham M. Sulakhe 1 , Mahesh B. Muluk 1 , Naziya N. M. A. Rehman 2 , Prashant P. Dixit 2 , Prafulla B. Choudhari 3 , Estharla Madhu Rekha 4 , Dharmarajan Sriram 4 , Kishan P. Haval 1
Affiliation
|
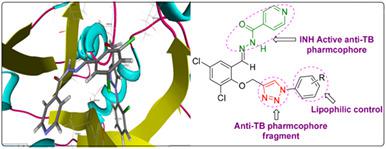
In the present study, a series of new isoniazid‐1,2,3‐triazole conjugates (5a‐k) was synthesized via click chemistry approach. The newly synthesized compounds were assessed for their in vitro antitubercular and antimicrobial activities. The compound 5g has displayed potent antitubercular activity against Mycobacterium tuberculosis H37Rv (Mtb) with MIC value 1.56 μg/mL. The active compounds were screened for their cytotoxicity profile by MTT assay against RAW 264.7 cell line. The four compounds have shown good in vitro antimicrobial activities against both antibacterial and antifungal pathogens. A molecular docking study was accomplished to identify the probable mode of action of synthesized derivatives. These compounds have shown excellent binding affinity toward Enoyl‐acp reductase (INHA) and DNA gyrase.
中文翻译:

异烟肼,1,2,3-三唑共轭物的合成:抗结核,抗菌药物评估和分子对接研究
在本研究中,通过点击化学方法合成了一系列新的异烟肼,1,2,3-三唑共轭物(5a-k)。对新合成的化合物的体外抗结核和抗微生物活性进行了评估。化合物5g对MIC值为1.56μg/ mL的结核分枝杆菌H37Rv(Mtb)表现出有效的抗结核活性。通过针对RAW 264.7细胞系的MTT测定筛选活性化合物的细胞毒性谱。四种化合物在体外显示出良好的针对抗菌和抗真菌病原体的抗菌活性。完成了分子对接研究,以确定合成衍生物的可能作用方式。这些化合物对Enoyl-acp还原酶(INHA)和DNA促旋酶显示出极好的结合亲和力。
更新日期:2020-06-25
中文翻译:

异烟肼,1,2,3-三唑共轭物的合成:抗结核,抗菌药物评估和分子对接研究
在本研究中,通过点击化学方法合成了一系列新的异烟肼,1,2,3-三唑共轭物(5a-k)。对新合成的化合物的体外抗结核和抗微生物活性进行了评估。化合物5g对MIC值为1.56μg/ mL的结核分枝杆菌H37Rv(Mtb)表现出有效的抗结核活性。通过针对RAW 264.7细胞系的MTT测定筛选活性化合物的细胞毒性谱。四种化合物在体外显示出良好的针对抗菌和抗真菌病原体的抗菌活性。完成了分子对接研究,以确定合成衍生物的可能作用方式。这些化合物对Enoyl-acp还原酶(INHA)和DNA促旋酶显示出极好的结合亲和力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号