当前位置:
X-MOL 学术
›
Thermochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Determination of the complexation parameters of L-asparagine with some biologically active pyridine derivatives in aqueous solutions from calorimetric results
Thermochimica Acta ( IF 3.5 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.tca.2020.178704 Elena Yu. Tyunina , Olga N. Krutova , Alexandr I. Lytkin
Thermochimica Acta ( IF 3.5 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.tca.2020.178704 Elena Yu. Tyunina , Olga N. Krutova , Alexandr I. Lytkin
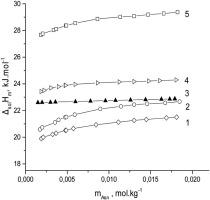
|
Abstract Thermodynamic functions (lgK, ΔcG, ΔcH, ΔcS) were determined by solution calorimetry for the complex formation process of L-asparagine in its zwitterionic form with pharmacologically active ligands, such as pyridoxine, nicotinic acid, isonicotinic acid and picolinic acid in aqueous solutions at 298.15 K. It was found that the complexation affinity of L-asparagine to the pyridine derivatives follows the order picolinic acid
中文翻译:

从量热结果确定 L-天冬酰胺与某些生物活性吡啶衍生物在水溶液中的络合参数
摘要 热力学函数 (lgK, ΔcG, ΔcH, ΔcS) 通过溶液量热法确定两性离子形式的 L-天冬酰胺与药理活性配体(如水溶液中的吡哆醇、烟酸、异烟酸和吡啶甲酸)的复合形成过程在 298.15 K. 发现 L-天冬酰胺与吡啶衍生物的络合亲和力遵循吡啶甲酸的顺序
更新日期:2020-08-01
中文翻译:

从量热结果确定 L-天冬酰胺与某些生物活性吡啶衍生物在水溶液中的络合参数
摘要 热力学函数 (lgK, ΔcG, ΔcH, ΔcS) 通过溶液量热法确定两性离子形式的 L-天冬酰胺与药理活性配体(如水溶液中的吡哆醇、烟酸、异烟酸和吡啶甲酸)的复合形成过程在 298.15 K. 发现 L-天冬酰胺与吡啶衍生物的络合亲和力遵循吡啶甲酸的顺序



























 京公网安备 11010802027423号
京公网安备 11010802027423号