iScience ( IF 4.6 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.isci.2020.101299 Eiichi Hashimoto 1 , Shota Okuno 1 , Shoshiro Hirayama 1 , Yoshiyuki Arata 1 , Tsuyoshi Goto 1 , Hidetaka Kosako 2 , Jun Hamazaki 1 , Shigeo Murata 1
|
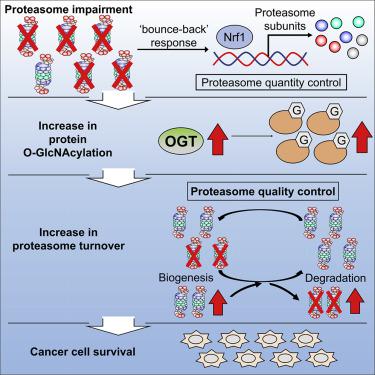
The proteasome is a therapeutic target in cancer, but resistance to proteasome inhibitors often develops owing to the induction of compensatory pathways. Through a genome-wide siRNA screen combined with RNA sequencing analysis, we identified hexokinase and downstream O-GlcNAcylation as cell survival factors under proteasome impairment. The inhibition of O-GlcNAcylation synergistically induced massive cell death in combination with proteasome inhibition. We further demonstrated that O-GlcNAcylation was indispensable for maintaining proteasome activity by enhancing biogenesis as well as proteasome degradation in a manner independent of Nrf1, a well-known compensatory transcription factor that upregulates proteasome gene expression. Our results identify a pathway that maintains proteasome function under proteasome impairment, providing potential targets for cancer therapy.
中文翻译:

增强的O-GlcNAcylation通过促进癌细胞中的蛋白酶体转换来介导蛋白酶体损伤下的细胞保护作用。
蛋白酶体是癌症的治疗靶标,但是由于诱导了补偿性途径,因此对蛋白酶体抑制剂的抗药性经常发展。通过全基因组的siRNA筛选与RNA测序分析相结合,我们确定了己糖激酶和下游O-GlcNAcylation是蛋白酶体损伤下的细胞存活因子。O-GlcNAcylation的抑制协同蛋白酶体抑制协同诱导大量细胞死亡。我们进一步证明O-GlcNAcylation是通过以不依赖于Nrf1(一种上调蛋白酶体基因表达的众所周知的补偿性转录因子)的方式增强生物发生和蛋白酶体降解来维持蛋白酶体活性所不可或缺的。我们的结果确定了在蛋白酶体损伤下维持蛋白酶体功能的途径,











































 京公网安备 11010802027423号
京公网安备 11010802027423号