Chemosphere ( IF 8.8 ) Pub Date : 2020-06-25 , DOI: 10.1016/j.chemosphere.2020.127331 Zhezheng Ding 1 , Yayi Yi 1 , Wenxing Wang 1 , Qingzhu Zhang 1
|
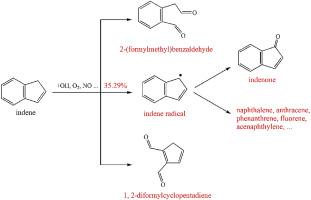
The atmospheric degradation of polycyclic aromatic hydrocarbons (PAHs) can generate organic pollutants that contribute to the formation of secondary organic aerosols (SOAs) and exacerbate their carcinogenicity. Indene is an example of styrene-like bicyclic hydrocarbons that are not fully aromatic. The OH-initiated atmospheric oxidation of indene in the presence of O2 and NO was investigated using quantum chemical methods at M06–2X/6–311++G(3df,2p)//M06–2X/6-311+G(d,p) level. The oxidation products are oxygenated polycyclic aromatic hydrocarbons (OPAHs) containing hydroxyindene, indenone, dialdehydes and 2-(formylmethyl)benzaldehyde. Calculation results showed that 7-indene radical, which is the precursor of various PAHs, has a high production ratio that is 35.29% in the initial reaction, indicating that the OH-initiated oxidation increase the environmental risks of indene in the atmosphere. The rate constants for the crucial elementary reactions were calculated based on Rice-Ramsperger-Kassel-Marcus (RRKM) theory. The overall rate constant of the initial reaction is calculated to be 1.04 × 10−10 cm3 molecule−1 s−1 and the atmospheric lifetime of indene is determined as 2.74 h. This work provides a comprehensive understanding on the oxidation mechanisms of indene and the findings could help to clarify the fate of indene in the atmosphere.
中文翻译:

在O2和NO存在下由OH自由基引发的茚的大气氧化:机理和动力学研究。
大气中多环芳烃(PAHs)的降解会产生有机污染物,这些有机污染物有助于形成次级有机气溶胶(SOA),并加剧其致癌性。茚是不完全芳族的苯乙烯状双环烃的实例。O 2存在下OH引发的茚的大气氧化使用M06–2X / 6–311 ++ G(3df,2p)// M06–2X / 6-311 + G(d,p)水平的量子化学方法研究了NO。氧化产物是含羟基茚,茚满酮,二醛和2-(甲酰甲基)苯甲醛的氧化多环芳烃(OPAH)。计算结果表明,作为多种PAHs的前体的7茚基自由基的高产率在初始反应中为35.29%,这表明OH引发的氧化增加了大气中茚的环境风险。根据赖斯-拉姆斯伯格-卡塞尔-马库斯(RRKM)理论计算关键的基本反应的速率常数。初始反应的总速率常数经计算为1.04×10 -10 cm 3分子-1 s-1,并且茚的大气寿命被确定为2.74h。这项工作对茚的氧化机理提供了全面的了解,这些发现有助于阐明大气中茚的命运。



























 京公网安备 11010802027423号
京公网安备 11010802027423号