Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 4.2 ) Pub Date : 2020-06-25 , DOI: 10.1016/j.bbadis.2020.165883 Yu Zhong 1 , Kabhilan Mohan 2 , Jinpeng Liu 3 , Ahmad Al-Attar 4 , Penghui Lin 4 , Robert M Flight 5 , Qiushi Sun 4 , Marc O Warmoes 4 , Rahul R Deshpande 4 , Huijuan Liu 1 , Kyung Sik Jung 2 , Mihail I Mitov 3 , Nianwei Lin 6 , D Allan Butterfield 7 , Shuyan Lu 6 , Jinze Liu 8 , Hunter N B Moseley 9 , Teresa W M Fan 10 , Mark E Kleinman 2 , Qing Jun Wang 11
|
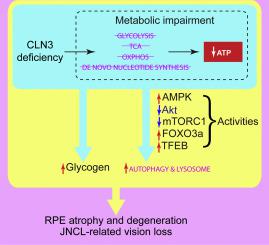
Juvenile neuronal ceroid lipofuscinosis (JNCL, aka. juvenile Batten disease or CLN3 disease) is a lysosomal storage disease characterized by progressive blindness, seizures, cognitive and motor failures, and premature death. JNCL is caused by mutations in the Ceroid Lipofuscinosis, Neuronal 3 (CLN3) gene, whose function is unclear. Although traditionally considered a neurodegenerative disease, CLN3 disease displays eye-specific effects: JNCL often first presents as vision loss; and vision loss has also been reported in non-syndromic CLN3 disease. Here we described the roles of CLN3 protein in maintaining healthy retinal pigment epithelium (RPE) and normal vision. Using electroretinogram, fundoscopy and microscopy, we showed impaired visual function, retinal autofluorescent lesions, and RPE disintegration and metaplasia/hyperplasia in a Cln3 ~ 1 kb-deletion mouse model [1] on C57BL/6J backgroun. Utilizing a combination of biochemical analyses, RNA-Seq, Seahorse XF bioenergetic analysis, and Stable Isotope Resolved Metabolomics (SIRM), we further demonstrated that loss of CLN3 increased autophagic flux, suppressed mTORC1 and Akt activities, enhanced AMPK activity, and up-regulated gene expression of the autophagy-lysosomal system in RPE-1 cells, suggesting autophagy induction. This CLN3 deficiency induced autophagy induction coincided with decreased mitochondrial oxygen consumption, glycolysis, the tricarboxylic acid (TCA) cycle, and ATP production. We also report for the first time that loss of CLN3 led to glycogen accumulation despite of impaired glycogen synthesis. Our comprehensive analyses shed light on how loss of CLN3 affect autophagy and metabolism. This work suggests possible links among metabolic impairment, autophagy induction and lysosomal storage, as well as between RPE atrophy/degeneration and vision loss in JNCL.
中文翻译:

幼年神经元蜡样脂褐质沉着症中突变的基因 CLN3 的缺失导致视网膜色素上皮细胞的代谢障碍和自噬诱导。
幼年神经元蜡样脂褐质沉积症(JNCL,又名幼年巴顿病或 CLN3 病)是一种溶酶体贮积病,其特征是进行性失明、癫痫发作、认知和运动障碍以及过早死亡。JNCL 是由蜡样脂褐质沉积症、神经元 3 ( CLN3) 基因,其功能尚不清楚。虽然传统上被认为是一种神经退行性疾病,但 CLN3 疾病表现出特定于眼睛的影响:JNCL 通常首先表现为视力丧失;在非综合征性 CLN3 疾病中也有视力丧失的报道。在这里,我们描述了 CLN3 蛋白在维持健康的视网膜色素上皮 (RPE) 和正常视力中的作用。使用视网膜电图、眼底镜检查和显微镜检查,我们在 C57BL/6J 背景上的Cln3 ~ 1 kb 缺失小鼠模型 [1] 中显示出视觉功能受损、视网膜自体荧光病变以及 RPE 崩解和化生/增生。利用生化分析、RNA-Seq、Seahorse XF 生物能量分析和稳定同位素解析代谢组学 (SIRM) 的组合,我们进一步证明了CLN3的丢失增加自噬通量,抑制 mTORC1 和 Akt 活性,增强 AMPK 活性,并上调 RPE-1 细胞中自噬-溶酶体系统的基因表达,表明自噬诱导。这种 CLN3 缺乏诱导的自噬诱导与线粒体耗氧量、糖酵解、三羧酸 (TCA) 循环和 ATP 产生的减少相吻合。我们还首次报告尽管糖原合成受损,但 CLN3 的缺失导致糖原积累。我们的综合分析揭示了CLN3的缺失如何影响自噬和新陈代谢。这项工作表明代谢障碍、自噬诱导和溶酶体储存之间可能存在联系,以及 JNCL 中 RPE 萎缩/变性和视力丧失之间可能存在联系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号