当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and theoretical insight into the adsorption of phenol and 2,4-dinitrophenol onto Tithonia diversifolia activated carbon
Applied Surface Science ( IF 6.3 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.apsusc.2020.147046 Aola Supong , Parimal Chandra Bhomick , Rituparna Karmaker , Soremo L. Ezung , Latonglila Jamir , Upasana Bora Sinha , Dipak Sinha
Applied Surface Science ( IF 6.3 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.apsusc.2020.147046 Aola Supong , Parimal Chandra Bhomick , Rituparna Karmaker , Soremo L. Ezung , Latonglila Jamir , Upasana Bora Sinha , Dipak Sinha
|
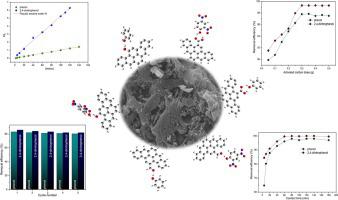
Abstract Adsorption of phenol and 2,4-dinitrophenol onto Tithonia diversifolia activated carbon (0.445 cm-3g−1 pore volume and 854.44 m2g−1 BET surface area) were studied by the experimental batch method and theoretical density functional theory. A maximum of 99.98% and 97.81% removal efficiency was attained for phenol and 2,4-dinitrophenol respectively at optimised conditions. The pseudo-second-order model described the adsorption kinetics well, while the Langmuir model best-elucidated the adsorption isotherm with a maximum adsorption capacity of 50.552 mg g−1 for phenol and 42.607 mg g−1 for 2,4-dinitrophenol. According to the calculated thermodynamic parameters, the adsorption process was spontaneous and endothermic. The process performance was further validated using real wastewater, and the removal efficiency of 84–90% was attained for both analytes. Theoretical investigations through density functional theory calculations suggested that the presence of oxygenated functional group on activated carbon surface decreased the adsorbate-adsorbent interaction. Meanwhile, a comparative study of the adsorption energies of various interactions indicated that phenol interacted more strongly with the activated carbon as compared to DNP. Regeneration studies indicated the reusability of the exhausted carbon up to the fifth cycle with significant removal efficiency.
中文翻译:

苯酚和 2,4-二硝基苯酚在 Tithonia diversifolia 活性炭上吸附的实验和理论见解
摘要 通过实验分批法和理论密度泛函理论研究了苯酚和2,4-二硝基苯酚在二氧化钛活性炭(0.445 cm-3g-1 孔体积和854.44 m2g-1 BET 表面积)上的吸附。在优化条件下,苯酚和 2,4-二硝基苯酚的去除效率分别达到 99.98% 和 97.81%。伪二级模型很好地描述了吸附动力学,而 Langmuir 模型最好地阐明了吸附等温线,苯酚的最大吸附容量为 50.552 mg g-1,2,4-二硝基苯酚的最大吸附容量为 42.607 mg g-1。根据计算的热力学参数,吸附过程是自发吸热的。使用真实废水进一步验证了工艺性能,两种分析物的去除效率都达到了 84-90%。通过密度泛函理论计算的理论研究表明,活性炭表面氧化官能团的存在降低了吸附质 - 吸附剂相互作用。同时,对各种相互作用的吸附能的比较研究表明,与 DNP 相比,苯酚与活性炭的相互作用更强。再生研究表明,排出的碳可重复使用到第五个循环,并具有显着的去除效率。对各种相互作用的吸附能的比较研究表明,与 DNP 相比,苯酚与活性炭的相互作用更强。再生研究表明,排出的碳可重复使用到第五个循环,并具有显着的去除效率。对各种相互作用的吸附能的比较研究表明,与 DNP 相比,苯酚与活性炭的相互作用更强。再生研究表明,排出的碳可重复使用到第五个循环,并具有显着的去除效率。
更新日期:2020-11-01
中文翻译:

苯酚和 2,4-二硝基苯酚在 Tithonia diversifolia 活性炭上吸附的实验和理论见解
摘要 通过实验分批法和理论密度泛函理论研究了苯酚和2,4-二硝基苯酚在二氧化钛活性炭(0.445 cm-3g-1 孔体积和854.44 m2g-1 BET 表面积)上的吸附。在优化条件下,苯酚和 2,4-二硝基苯酚的去除效率分别达到 99.98% 和 97.81%。伪二级模型很好地描述了吸附动力学,而 Langmuir 模型最好地阐明了吸附等温线,苯酚的最大吸附容量为 50.552 mg g-1,2,4-二硝基苯酚的最大吸附容量为 42.607 mg g-1。根据计算的热力学参数,吸附过程是自发吸热的。使用真实废水进一步验证了工艺性能,两种分析物的去除效率都达到了 84-90%。通过密度泛函理论计算的理论研究表明,活性炭表面氧化官能团的存在降低了吸附质 - 吸附剂相互作用。同时,对各种相互作用的吸附能的比较研究表明,与 DNP 相比,苯酚与活性炭的相互作用更强。再生研究表明,排出的碳可重复使用到第五个循环,并具有显着的去除效率。对各种相互作用的吸附能的比较研究表明,与 DNP 相比,苯酚与活性炭的相互作用更强。再生研究表明,排出的碳可重复使用到第五个循环,并具有显着的去除效率。对各种相互作用的吸附能的比较研究表明,与 DNP 相比,苯酚与活性炭的相互作用更强。再生研究表明,排出的碳可重复使用到第五个循环,并具有显着的去除效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号