当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of aziridines with multiple chiral substitutions by copper-catalyzed diastereoselective radical aminotrifluoromethylation of alkenes
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-06-24 , DOI: 10.1039/d0qo00603c Shuanglin Qin 1, 2, 3, 4, 5 , Linbin Yao 5, 6, 7, 8, 9 , Yunhao Luo 1, 2, 3, 4, 5 , Tongtong Liu 1, 2, 3, 4, 5 , Jiayuan Xu 5, 6, 7, 8, 9 , Yue Sun 1, 2, 3, 4, 5 , Ning Wang 1, 2, 3, 4, 5 , Jun Yan 5, 10, 11, 12 , Bencan Tang 5, 6, 7, 8, 9 , Guang Yang 1, 2, 3, 4, 5 , Cheng Yang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-06-24 , DOI: 10.1039/d0qo00603c Shuanglin Qin 1, 2, 3, 4, 5 , Linbin Yao 5, 6, 7, 8, 9 , Yunhao Luo 1, 2, 3, 4, 5 , Tongtong Liu 1, 2, 3, 4, 5 , Jiayuan Xu 5, 6, 7, 8, 9 , Yue Sun 1, 2, 3, 4, 5 , Ning Wang 1, 2, 3, 4, 5 , Jun Yan 5, 10, 11, 12 , Bencan Tang 5, 6, 7, 8, 9 , Guang Yang 1, 2, 3, 4, 5 , Cheng Yang 1, 2, 3, 4, 5
Affiliation
|
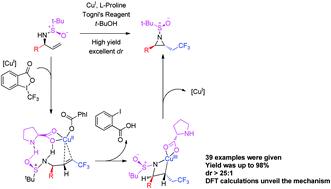
A one-step catalytic and diastereoselective method for the synthesis of aziridines possessing multiple chiral substitutions by radical aminotrifluoromethylation of alkenes has been developed for the first time. The reaction utilizes a Cu(I)/L-proline complex as a catalyst and a chiral sulfinamide group performs the role of nucleophile and chiral directing group. This synthetic strategy provides one-step access to a wide variety of substituted aziridines with good chemical yield, excellent diastereoselectivity and broad functional group tolerance. A possible reaction mechanism is proposed based on DFT calculations. The method should be useful for the rapid synthesis of chiral CF3-containing building blocks and novel drug molecules.
中文翻译:

铜催化烯烃的非对映选择性自由基氨基三氟甲基化反应合成具有多个手性取代基的氮丙啶
首次开发了一种通过烯烃的自由基氨基三氟甲基化合成具有多个手性取代基的氮丙啶的一步催化和非对映选择性方法。该反应利用Cu(I)/ L-脯氨酸配合物作为催化剂,并且手性亚磺酰胺基团发挥亲核试剂和手性指导基团的作用。这种合成策略可一步一步获得各种取代的氮丙啶,具有良好的化学收率,出色的非对映选择性和广泛的官能团耐受性。基于DFT计算,提出了一种可能的反应机理。该方法对于快速合成含手性CF 3的结构单元和新型药物分子应该是有用的。
更新日期:2020-06-24
中文翻译:

铜催化烯烃的非对映选择性自由基氨基三氟甲基化反应合成具有多个手性取代基的氮丙啶
首次开发了一种通过烯烃的自由基氨基三氟甲基化合成具有多个手性取代基的氮丙啶的一步催化和非对映选择性方法。该反应利用Cu(I)/ L-脯氨酸配合物作为催化剂,并且手性亚磺酰胺基团发挥亲核试剂和手性指导基团的作用。这种合成策略可一步一步获得各种取代的氮丙啶,具有良好的化学收率,出色的非对映选择性和广泛的官能团耐受性。基于DFT计算,提出了一种可能的反应机理。该方法对于快速合成含手性CF 3的结构单元和新型药物分子应该是有用的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号