当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Systematic Theoretical Kinetics Analysis for the Waddington Mechanism in the Low-Temperature Oxidation of Butene and Butanol Isomers.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.jpca.0c03515 Yang Li 1 , Qian Zhao 2 , Yingjia Zhang 2 , Zuohua Huang 2 , S Mani Sarathy 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.jpca.0c03515 Yang Li 1 , Qian Zhao 2 , Yingjia Zhang 2 , Zuohua Huang 2 , S Mani Sarathy 1
Affiliation
|
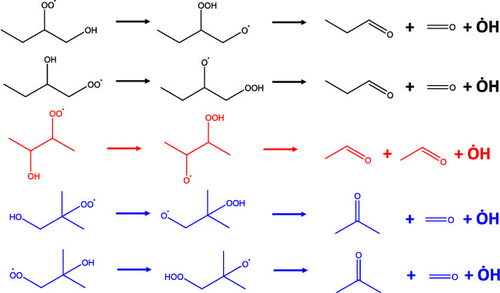
The Waddington mechanism, or the Waddington-type reaction pathway, is crucial for low-temperature oxidation of both alkenes and alcohols. In this study, the Waddington mechanism in the oxidation chemistry of butene and butanol isomers was systematically investigated. Fundamental quantum chemical calculations were conducted for the rate constants and thermodynamic properties of the reactions and species in this mechanism. Calculations were performed using two different ab initio solvers: Gaussian 09 and Orca 4.0.0, and two different kinetic solvers: PAPR and MultiWell, comprehensively. Temperature- and pressure-dependent rate constants were performed based on the transition state theory, associated with the Rice Ramsperger Kassel Marcus and master equation theories. Temperature-dependent thermochemistry (enthalpies of formation, entropy, and heat capacity) of all major species was also conducted, based on the statistical thermodynamics. Of the two types of reaction, dissociation reactions were significantly faster than isomerization reactions, while the rate constants of both reactions converged toward higher temperatures. In comparison, between two ab initio solvers, the barrier height difference among all isomerization and dissociation reactions was about 2 and 0.5 kcal/mol, respectively, resulting in less than 50%, and a factor of 2–10 differences for the predicted rate coefficients of the two reaction types, respectively. Comparing the two kinetic solvers, the rate constants of the isomerization reactions showed less than a 32% difference, while the rate of one dissociation reaction (P1 ↔ WDT12) exhibited 1–2 orders of magnitude discrepancy. Compared with results from the literature, both reaction rate coefficients (R4 and R5 reaction systems) and species’ thermochemistry (all closed shell molecules and open shell radicals R4 and R5) showed good agreement with the corresponding values obtained from the literature. All calculated results can be directly used for the chemical kinetic model development of butene and butanol isomer oxidation.
中文翻译:

丁烯和丁醇异构体的低温氧化中沃丁顿机理的系统理论动力学分析。
Waddington机制或Waddington型反应途径对于烯烃和醇类的低温氧化至关重要。在这项研究中,系统研究了Waddington在丁烯和丁醇异构体氧化化学中的机理。在此机理中,对反应和物种的速率常数和热力学性质进行了基本的量子化学计算。使用两个不同的从头算进行计算求解器:Gaussian 09和Orca 4.0.0,以及两个不同的动力学求解器:PAPR和MultiWell。基于温度和压力的速率常数是基于与赖斯·兰斯珀格·卡塞尔·马库斯(Rice Ramsperger Kassel Marcus)和主方程理论相关的过渡态理论进行的。根据统计热力学,还对所有主要物种进行了温度依赖性的热化学反应(形成焓,熵和热容)。在两种类型的反应中,解离反应明显快于异构化反应,而两种反应的速率常数均趋于较高的温度。相比之下,两个从头算起求解器中,所有异构化和解离反应之间的势垒高度差分别约为2和0.5 kcal / mol,导致小于50%,并且两种反应类型的预测速率系数分别相差2-10倍。比较两个动力学求解器,异构化反应的速率常数差异小于32%,而一个解离反应的速率(P1↔WDT12)表现出1-2个数量级的差异。与文献结果相比,反应速率系数(R4和R5反应体系)和物质的热化学性质(所有封闭壳分子和开放壳自由基R4和R5)都与文献中获得的相应值吻合良好。
更新日期:2020-07-09
中文翻译:

丁烯和丁醇异构体的低温氧化中沃丁顿机理的系统理论动力学分析。
Waddington机制或Waddington型反应途径对于烯烃和醇类的低温氧化至关重要。在这项研究中,系统研究了Waddington在丁烯和丁醇异构体氧化化学中的机理。在此机理中,对反应和物种的速率常数和热力学性质进行了基本的量子化学计算。使用两个不同的从头算进行计算求解器:Gaussian 09和Orca 4.0.0,以及两个不同的动力学求解器:PAPR和MultiWell。基于温度和压力的速率常数是基于与赖斯·兰斯珀格·卡塞尔·马库斯(Rice Ramsperger Kassel Marcus)和主方程理论相关的过渡态理论进行的。根据统计热力学,还对所有主要物种进行了温度依赖性的热化学反应(形成焓,熵和热容)。在两种类型的反应中,解离反应明显快于异构化反应,而两种反应的速率常数均趋于较高的温度。相比之下,两个从头算起求解器中,所有异构化和解离反应之间的势垒高度差分别约为2和0.5 kcal / mol,导致小于50%,并且两种反应类型的预测速率系数分别相差2-10倍。比较两个动力学求解器,异构化反应的速率常数差异小于32%,而一个解离反应的速率(P1↔WDT12)表现出1-2个数量级的差异。与文献结果相比,反应速率系数(R4和R5反应体系)和物质的热化学性质(所有封闭壳分子和开放壳自由基R4和R5)都与文献中获得的相应值吻合良好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号