当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lipid Compositions in Infant Formulas Affect the Solubilization of Antimalarial Drugs Artefenomel (OZ439) and Ferroquine during Digestion.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.molpharmaceut.0c00475 Malinda Salim 1 , Gisela Ramirez 1 , Kang-Yu Peng 1 , Andrew J Clulow 1 , Adrian Hawley 2 , Hanu Ramachandruni 3 , Stephane Beilles 4 , Ben J Boyd 1, 5
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.molpharmaceut.0c00475 Malinda Salim 1 , Gisela Ramirez 1 , Kang-Yu Peng 1 , Andrew J Clulow 1 , Adrian Hawley 2 , Hanu Ramachandruni 3 , Stephane Beilles 4 , Ben J Boyd 1, 5
Affiliation
|
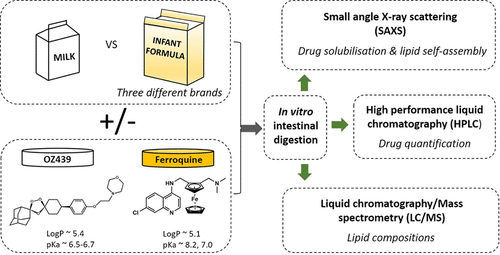
Recent studies have shown that the solubilization of two antimalarial drug candidates, artefenomel (OZ439) and ferroquine (FQ), designed to provide a single-dose combination therapy for uncomplicated malaria can be enhanced using milk as a lipid-based formulation. However, milk as an excipient faces significant quality and regulatory hurdles. We therefore have investigated infant formula as a potential alternative formulation approach. The significance of the lipid species present in a formula with different lipid compositions upon the solubilization of OZ439 and FQ during digestion has been investigated. Synchrotron small-angle X-ray scattering was used to measure the diffraction from a dispersed drug during digestion and thereby determine the extent of drug solubilization. High-performance liquid chromatography was used to quantify the amount of drug partitioned into the digested lipid phases. Our results show that both the lipid species and the amount of lipids administered were key determinants for the solubilization of OZ439, while the solubilization of FQ was independent of the lipid composition. Infant formulas could therefore be designed and used as milk substitutes to tailor the desired level of drug solubilization while circumventing the variability of components in naturally derived milk. The enhanced solubilization of OZ439 was achieved during the digestion of medium-chain triacylglycerols (MCT), indicating the potential applicability of MCT-fortified infant formula powder as a lipid-based formulation for the oral delivery of OZ439 and FQ.
中文翻译:

婴儿配方食品中的脂质成分会影响消化过程中抗疟药Artefenomel(OZ439)和四氢呋喃的增溶作用。
最近的研究表明,使用牛奶作为基于脂质的制剂,可以增强两种抗疟药候选药物Artefenomel(OZ439)和Ferroquine(FQ)的增溶作用,这些药物旨在为单纯性疟疾提供单剂量联合治疗。然而,作为赋形剂的牛奶面临着明显的质量和法规障碍。因此,我们已经研究了婴儿配方奶粉作为一种潜在的替代配方方法。研究了在消化过程中OZ439和FQ增溶后,具有不同脂质组成的配方中存在的脂质种类的重要性。同步加速器小角度X射线散射用于测量消化过程中分散药物的衍射,从而确定药物溶解的程度。高效液相色谱法用于定量分配到消化的脂质相中的药物量。我们的结果表明,脂质种类和所施用的脂质量都是OZ439增溶的关键决定因素,而FQ的增溶与脂质组成无关。因此,可以设计婴儿配方奶粉并将其用作牛奶代用品,以调节所需的药物增溶水平,同时规避天然奶中成分的可变性。OZ439的增溶作用是在中链三酰基甘油(MCT)的消化过程中实现的,表明MCT强化的婴儿配方奶粉作为基于脂质的配方可用于OZ439和FQ的口服给药。我们的结果表明,脂质种类和所施用的脂质量都是OZ439增溶的关键决定因素,而FQ的增溶与脂质组成无关。因此,可以设计婴儿配方奶粉并将其用作牛奶代用品,以调节所需的药物增溶水平,同时规避天然奶中成分的可变性。OZ439的增溶作用是在中链三酰基甘油(MCT)的消化过程中实现的,表明MCT强化的婴儿配方奶粉作为基于脂质的配方可用于OZ439和FQ的口服给药。我们的结果表明,脂质种类和所施用的脂质量都是OZ439增溶的关键决定因素,而FQ的增溶与脂质组成无关。因此,可以设计婴儿配方奶粉并将其用作牛奶代用品,以调节所需的药物增溶水平,同时规避天然牛奶中各成分的可变性。OZ439的增溶作用是在中链三酰基甘油(MCT)的消化过程中实现的,表明MCT强化的婴儿配方奶粉作为基于脂质的配方可用于OZ439和FQ的口服给药。因此,可以设计婴儿配方奶粉并将其用作牛奶代用品,以调节所需的药物增溶水平,同时规避天然牛奶中成分的可变性。OZ439的增溶作用在中链三酰基甘油(MCT)的消化过程中得以实现,这表明MCT强化的婴儿配方粉作为基于脂质的配方可用于OZ439和FQ的口服给药。因此,可以设计婴儿配方奶粉并将其用作牛奶代用品,以调节所需的药物增溶水平,同时规避天然牛奶中各成分的可变性。OZ439的增溶作用是在中链三酰基甘油(MCT)的消化过程中实现的,表明MCT强化的婴儿配方奶粉作为基于脂质的配方可用于OZ439和FQ的口服给药。
更新日期:2020-07-06
中文翻译:

婴儿配方食品中的脂质成分会影响消化过程中抗疟药Artefenomel(OZ439)和四氢呋喃的增溶作用。
最近的研究表明,使用牛奶作为基于脂质的制剂,可以增强两种抗疟药候选药物Artefenomel(OZ439)和Ferroquine(FQ)的增溶作用,这些药物旨在为单纯性疟疾提供单剂量联合治疗。然而,作为赋形剂的牛奶面临着明显的质量和法规障碍。因此,我们已经研究了婴儿配方奶粉作为一种潜在的替代配方方法。研究了在消化过程中OZ439和FQ增溶后,具有不同脂质组成的配方中存在的脂质种类的重要性。同步加速器小角度X射线散射用于测量消化过程中分散药物的衍射,从而确定药物溶解的程度。高效液相色谱法用于定量分配到消化的脂质相中的药物量。我们的结果表明,脂质种类和所施用的脂质量都是OZ439增溶的关键决定因素,而FQ的增溶与脂质组成无关。因此,可以设计婴儿配方奶粉并将其用作牛奶代用品,以调节所需的药物增溶水平,同时规避天然奶中成分的可变性。OZ439的增溶作用是在中链三酰基甘油(MCT)的消化过程中实现的,表明MCT强化的婴儿配方奶粉作为基于脂质的配方可用于OZ439和FQ的口服给药。我们的结果表明,脂质种类和所施用的脂质量都是OZ439增溶的关键决定因素,而FQ的增溶与脂质组成无关。因此,可以设计婴儿配方奶粉并将其用作牛奶代用品,以调节所需的药物增溶水平,同时规避天然奶中成分的可变性。OZ439的增溶作用是在中链三酰基甘油(MCT)的消化过程中实现的,表明MCT强化的婴儿配方奶粉作为基于脂质的配方可用于OZ439和FQ的口服给药。我们的结果表明,脂质种类和所施用的脂质量都是OZ439增溶的关键决定因素,而FQ的增溶与脂质组成无关。因此,可以设计婴儿配方奶粉并将其用作牛奶代用品,以调节所需的药物增溶水平,同时规避天然牛奶中各成分的可变性。OZ439的增溶作用是在中链三酰基甘油(MCT)的消化过程中实现的,表明MCT强化的婴儿配方奶粉作为基于脂质的配方可用于OZ439和FQ的口服给药。因此,可以设计婴儿配方奶粉并将其用作牛奶代用品,以调节所需的药物增溶水平,同时规避天然牛奶中成分的可变性。OZ439的增溶作用在中链三酰基甘油(MCT)的消化过程中得以实现,这表明MCT强化的婴儿配方粉作为基于脂质的配方可用于OZ439和FQ的口服给药。因此,可以设计婴儿配方奶粉并将其用作牛奶代用品,以调节所需的药物增溶水平,同时规避天然牛奶中各成分的可变性。OZ439的增溶作用是在中链三酰基甘油(MCT)的消化过程中实现的,表明MCT强化的婴儿配方奶粉作为基于脂质的配方可用于OZ439和FQ的口服给药。











































 京公网安备 11010802027423号
京公网安备 11010802027423号