当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
HIOx-IONO2 Dynamics at the Air-Water Interface: Revealing the Existence of a Halogen Bond at the Atmospheric Aerosol Surface
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-24 , DOI: 10.1021/jacs.0c05232 Manoj Kumar 1 , Tarek Trabelsi 1 , Juan Carlos Gómez Martín 2 , Alfonso Saiz-Lopez 3 , Joseph S Francisco 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-24 , DOI: 10.1021/jacs.0c05232 Manoj Kumar 1 , Tarek Trabelsi 1 , Juan Carlos Gómez Martín 2 , Alfonso Saiz-Lopez 3 , Joseph S Francisco 1
Affiliation
|
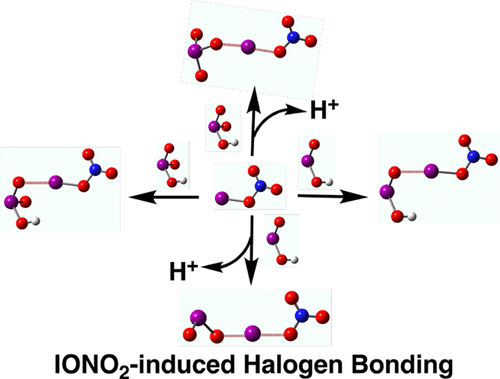
Iodine is enriched in marine aerosols, particularly in coastal mid-latitude atmospheric environments, where it initiates the formation of new aerosol particles with iodic acid (HIO3) composition. However, particle formation in polluted and semi-polluted locations is inhibited when the iodine monoxide radical (IO) is intercepted by NO2 to form the iodine nitrate (IONO2). The primary fate of IONO2 is believed to be, besides photolysis, uptake by aerosol surfaces, leading to particulate iodine activation. Herein we have performed Born Oppenheimer molecular dynamics (BOMD) simulations and gas-phase quantum chemical calculations to study the iodine acids-iodine nitrate (HIOx(x = 2 and 3)-IONO2) dynamics at the air-water interface modeled by a water droplet of 191 water molecules. The results indicate that IONO2 does not react directly with these iodine acids, but forms an unu-sual kind of interaction with them within a few picoseconds, which is characterized as halogen bonding. The halogen bond-driven HIO3-IONO2 complex at the air-water interface undergoes deprotonation and exists as IO3--IONO2 anion whereas the HIO2-IONO2 complex does not exhibit any proton loss to the interfacial water molecules. The gas-phase quantum chemical calculations suggest that the HIO3-IONO2 and HIO2-IONO2 complexes have appreciable stabilization energies, which are significantly enhanced upon deprotonation of iodine acids, indicating that these halogen bonds are fairly stable. These IONO2-induced halogen bonds explain the rapid loss of IONO2 to background aerosol. Moreover, they appear to work against iodide formation. Thus, they may play an important role in enhancing the amount of atmospherically non-recyclable iodine (iodate) in marine aerosol.
中文翻译:

空气-水界面的 HIOx-IONO2 动力学:揭示大气气溶胶表面卤素键的存在
碘在海洋气溶胶中含量丰富,特别是在沿海中纬度大气环境中,它会引发新的具有碘酸 (HIO3) 成分的气溶胶颗粒的形成。然而,当一氧化碘自由基 (IO) 被 NO2 拦截形成硝酸碘 (IONO2) 时,污染和半污染位置的颗粒形成受到抑制。据信 IONO2 的主要归宿除了光解外,还被气溶胶表面吸收,导致微粒碘活化。在此,我们进行了 Born Oppenheimer 分子动力学 (BOMD) 模拟和气相量子化学计算,以研究由水模拟的空气-水界面处的碘酸-硝酸碘(HIOx(x = 2 和 3)-IONO2)动力学191个水分子的液滴。结果表明 IONO2 不直接与这些碘酸反应,而是在几皮秒内与它们形成一种不寻常的相互作用,其特征是卤素键。空气-水界面处的卤素键驱动的 HIO3-IONO2 复合物发生去质子化并以 IO3--IONO2 阴离子的形式存在,而 HIO2-IONO2 复合物不会对界面水分子表现出任何质子损失。气相量子化学计算表明,HIO3-IONO2 和 HIO2-IONO2 配合物具有可观的稳定能,在碘酸去质子化时显着增强,表明这些卤素键相当稳定。这些 IONO2 诱导的卤素键解释了 IONO2 快速流失到背景气溶胶中。此外,它们似乎对碘化物的形成起作用。因此,
更新日期:2020-06-24
中文翻译:

空气-水界面的 HIOx-IONO2 动力学:揭示大气气溶胶表面卤素键的存在
碘在海洋气溶胶中含量丰富,特别是在沿海中纬度大气环境中,它会引发新的具有碘酸 (HIO3) 成分的气溶胶颗粒的形成。然而,当一氧化碘自由基 (IO) 被 NO2 拦截形成硝酸碘 (IONO2) 时,污染和半污染位置的颗粒形成受到抑制。据信 IONO2 的主要归宿除了光解外,还被气溶胶表面吸收,导致微粒碘活化。在此,我们进行了 Born Oppenheimer 分子动力学 (BOMD) 模拟和气相量子化学计算,以研究由水模拟的空气-水界面处的碘酸-硝酸碘(HIOx(x = 2 和 3)-IONO2)动力学191个水分子的液滴。结果表明 IONO2 不直接与这些碘酸反应,而是在几皮秒内与它们形成一种不寻常的相互作用,其特征是卤素键。空气-水界面处的卤素键驱动的 HIO3-IONO2 复合物发生去质子化并以 IO3--IONO2 阴离子的形式存在,而 HIO2-IONO2 复合物不会对界面水分子表现出任何质子损失。气相量子化学计算表明,HIO3-IONO2 和 HIO2-IONO2 配合物具有可观的稳定能,在碘酸去质子化时显着增强,表明这些卤素键相当稳定。这些 IONO2 诱导的卤素键解释了 IONO2 快速流失到背景气溶胶中。此外,它们似乎对碘化物的形成起作用。因此,











































 京公网安备 11010802027423号
京公网安备 11010802027423号