当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Saturated Solubility and Thermodynamic Mixing Properties of 3,5-Dibromo-4-hydroxybenzaldehyde in 16 Individual Solvents at Elevated Temperatures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-24 , DOI: 10.1021/acs.jced.0c00376 Changfei Zhu 1 , Hao Yin 1 , Yanyan Zhou 1 , Hongkun Zhao 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-24 , DOI: 10.1021/acs.jced.0c00376 Changfei Zhu 1 , Hao Yin 1 , Yanyan Zhou 1 , Hongkun Zhao 1
Affiliation
|
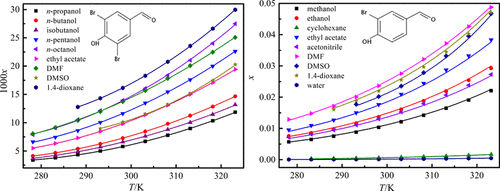
This work aimed at saturated solubility of 3,5-dibromo-4-hydroxybenzaldehyde in 16 individual solvents including n-pentanol, methanol, dimethylsulfoxide (DMSO), isobutanol, ethanol, 1,4-dioxane, n-propanol, ethylene glycol (EG), isopropanol, cyclohexane, n-butanol, n-octanol, ethyl acetate, water, N,N-dimethylformamide (DMF), and acetonitrile. Experiments were performed through a saturation shake-flask method within the temperature range of 278.15–323.15 K under ambient pressure of about p = 101.2 kPa. The solubility values in mole fraction of 3,5-dibromo-4-hydroxybenzaldehyde increased as the experimental temperature increased and are ranked as: 1,4-dioxane > (DMF, n-octanol) > n-pentanol > DMSO > ethyl acetate > n-butanol > isobutanol > n-propanol > isopropanol > (ethanol, EG) > acetonitrile > methanol > cyclohexane > water. The molecular interactions of solvent–solute and solvent–solvent were examined by way of linear solvation energy relationships to show the dependence of solvent descriptors on solubility behavior. Several equations and models, e.g., Wilson, λh, Apelblat, and NRTL, were employed to mathematically correlate the solubility data. The obtained values of maximum root-mean-square deviation and relative average deviation were 4.191 × 10–4 and 2.61 × 10–2, respectively. In general, for a particular solvent, the relative average deviation values obtained with the Apelblat equation were smaller than those obtained with the other models/equation. Also, the activity coefficient and reduced excess enthalpy under the condition of infinitesimal concentration along with mixing properties were obtained using the Wilson model.
中文翻译:

3,5-二溴-4-羟基苯甲醛在高温下在16种单独溶剂中的饱和溶解度和热力学混合特性
这项工作旨在将3,5-二溴-4-羟基苯甲醛在16种单独的溶剂中的饱和溶解度包括正戊醇,甲醇,二甲亚砜(DMSO),异丁醇,乙醇,1,4-二恶烷,正丙醇,乙二醇(EG ),异丙醇,环己烷,正丁醇,正辛醇,乙酸乙酯,水,N,N-二甲基甲酰胺(DMF)和乙腈。实验是通过饱和摇瓶法在278.15–323.15 K的温度范围内,环境压力约为p的条件下进行的。= 101.2 kPa。3,5-二溴-4-羟基苯甲醛的溶解度值以摩尔分数计随着实验温度的升高而增加,其排名为:1,4-二恶烷>(DMF,正辛醇)>正戊醇> DMSO>乙酸乙酯>正丁醇>异丁醇>正丙醇>异丙醇>(乙醇,EG)>乙腈>甲醇>环己烷>水。通过线性溶剂化能量关系检查了溶剂-溶质和溶剂-溶剂的分子相互作用,以显示溶剂描述符对溶解度行为的依赖性。一些公式和模型,例如,威尔逊,λ ^ h,Apelblat和NRTL用于数学关联溶解度数据。获得的最大均方根偏差和相对平均偏差分别为4.191×10 –4和2.61×10 –2。通常,对于特定溶剂,通过Apelblat方程获得的相对平均偏差值小于通过其他模型/方程式获得的相对平均偏差值。另外,使用威尔逊模型获得了在最小浓度下的活度系数和降低的过量焓以及混合性能。
更新日期:2020-07-09
中文翻译:

3,5-二溴-4-羟基苯甲醛在高温下在16种单独溶剂中的饱和溶解度和热力学混合特性
这项工作旨在将3,5-二溴-4-羟基苯甲醛在16种单独的溶剂中的饱和溶解度包括正戊醇,甲醇,二甲亚砜(DMSO),异丁醇,乙醇,1,4-二恶烷,正丙醇,乙二醇(EG ),异丙醇,环己烷,正丁醇,正辛醇,乙酸乙酯,水,N,N-二甲基甲酰胺(DMF)和乙腈。实验是通过饱和摇瓶法在278.15–323.15 K的温度范围内,环境压力约为p的条件下进行的。= 101.2 kPa。3,5-二溴-4-羟基苯甲醛的溶解度值以摩尔分数计随着实验温度的升高而增加,其排名为:1,4-二恶烷>(DMF,正辛醇)>正戊醇> DMSO>乙酸乙酯>正丁醇>异丁醇>正丙醇>异丙醇>(乙醇,EG)>乙腈>甲醇>环己烷>水。通过线性溶剂化能量关系检查了溶剂-溶质和溶剂-溶剂的分子相互作用,以显示溶剂描述符对溶解度行为的依赖性。一些公式和模型,例如,威尔逊,λ ^ h,Apelblat和NRTL用于数学关联溶解度数据。获得的最大均方根偏差和相对平均偏差分别为4.191×10 –4和2.61×10 –2。通常,对于特定溶剂,通过Apelblat方程获得的相对平均偏差值小于通过其他模型/方程式获得的相对平均偏差值。另外,使用威尔逊模型获得了在最小浓度下的活度系数和降低的过量焓以及混合性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号