当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cluster Formation and Its Role in the Elimination of Azeotrope of the Acetone–Methanol Mixture by Ionic Liquids
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-06-24 , DOI: 10.1021/acs.iecr.0c01292 Fang Chen , Long Zhang , Zhiping Liu , Guangren Yu
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-06-24 , DOI: 10.1021/acs.iecr.0c01292 Fang Chen , Long Zhang , Zhiping Liu , Guangren Yu
|
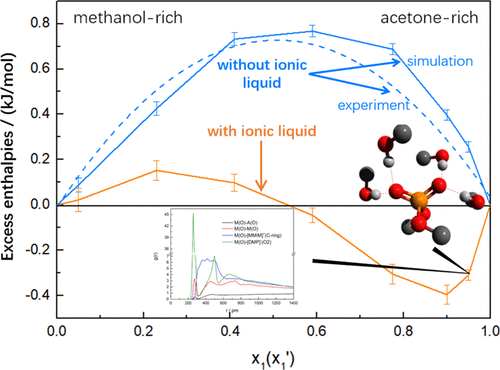
Ionic liquids (ILs) are promising entrainers in the separation of azeotropic systems with unique advantages, such as environmental benignity and flexible design. In this work, we carried out molecular dynamics (MD) simulations to elucidate the underlying mechanism in the separation of a typical azeotropic mixture, acetone (A)–methanol (M), using an efficient IL entrainer, 1,3-dimethylimidazolium dimethylphosphate ([MMIM][DMP]), which was proposed in our previous work. Upon the addition of the IL, the excess enthalpies decrease significantly, especially in the acetone-rich mixture. An analysis based on energy breakdown in MD simulations indicated that the interactions between methanol and [DMP]− play a major role in the elimination of the azeotrope. In terms of the radial distribution function, spatial distribution function, and combined distribution function, we discussed the microscopic structures to clarify various interactions among the mixtures. It was found that various clusters of dimensions less than 10 Å are formed by methanol and [DMP]−, in the acetone-rich mixture upon the addition of the IL. In addition, we distinguished the types of these clusters in terms of their different connection modes by the detailed information extracted in simulations. Results indicated that the interplay of two kinds of hydrogen bonds, that is, O–H(M)···O2(D) and O–H(M)···O(M), is responsible for the formation of these clusters. We provided important information to understand the mechanisms of azeotrope elimination by the IL, which is valuable to design new entrainers in the future.
中文翻译:

离子液体消除团簇形成及其在消除丙酮-甲醇混合物共沸物中的作用
离子液体(ILs)是分离共沸体系的有前途的夹带剂,具有独特的优势,例如环境友好性和灵活的设计。在这项工作中,我们进行了分子动力学(MD)模拟,以阐明使用高效的IL夹带剂1,3-二甲基咪唑鎓二甲基磷酸二甲酯(A)-甲醇(M)分离典型共沸混合物的基本机理。 [MMIM] [DMP]),这是在我们之前的工作中提出的。加入IL后,过量的焓显着降低,尤其是在富含丙酮的混合物中。指示基于能量击穿在MD模拟分析甲醇和之间的相互作用[DMP] -在消除共沸物方面起主要作用。在径向分布函数,空间分布函数和组合分布函数方面,我们讨论了微观结构以阐明混合物之间的各种相互作用。发现甲醇和[DMP] -形成了尺寸小于10Å的各种簇在加入IL后,在富含丙酮的混合物中溶解。此外,我们通过模拟中提取的详细信息,根据不同的连接模式区分了这些群集的类型。结果表明,O–H(M)···O2(D)和OH–H(M)···O(M)这两种氢键的相互作用是导致这些氢键形成的原因。集群。我们提供了重要的信息,以了解IL消除共沸物的机制,这对于将来设计新的夹带剂非常有价值。
更新日期:2020-07-22
中文翻译:

离子液体消除团簇形成及其在消除丙酮-甲醇混合物共沸物中的作用
离子液体(ILs)是分离共沸体系的有前途的夹带剂,具有独特的优势,例如环境友好性和灵活的设计。在这项工作中,我们进行了分子动力学(MD)模拟,以阐明使用高效的IL夹带剂1,3-二甲基咪唑鎓二甲基磷酸二甲酯(A)-甲醇(M)分离典型共沸混合物的基本机理。 [MMIM] [DMP]),这是在我们之前的工作中提出的。加入IL后,过量的焓显着降低,尤其是在富含丙酮的混合物中。指示基于能量击穿在MD模拟分析甲醇和之间的相互作用[DMP] -在消除共沸物方面起主要作用。在径向分布函数,空间分布函数和组合分布函数方面,我们讨论了微观结构以阐明混合物之间的各种相互作用。发现甲醇和[DMP] -形成了尺寸小于10Å的各种簇在加入IL后,在富含丙酮的混合物中溶解。此外,我们通过模拟中提取的详细信息,根据不同的连接模式区分了这些群集的类型。结果表明,O–H(M)···O2(D)和OH–H(M)···O(M)这两种氢键的相互作用是导致这些氢键形成的原因。集群。我们提供了重要的信息,以了解IL消除共沸物的机制,这对于将来设计新的夹带剂非常有价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号