当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Position-Dependent Effect of Guanine Base Damage and Mutations on Telomeric G-Quadruplex and Telomerase Extension.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-24 , DOI: 10.1021/acs.biochem.0c00434 Hui-Ting Lee 1 , Samantha Sanford 2 , Tapas Paul 1 , Joshua Choe 1 , Arindam Bose 2 , Patricia L Opresko 2 , Sua Myong 1, 3
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-24 , DOI: 10.1021/acs.biochem.0c00434 Hui-Ting Lee 1 , Samantha Sanford 2 , Tapas Paul 1 , Joshua Choe 1 , Arindam Bose 2 , Patricia L Opresko 2 , Sua Myong 1, 3
Affiliation
|
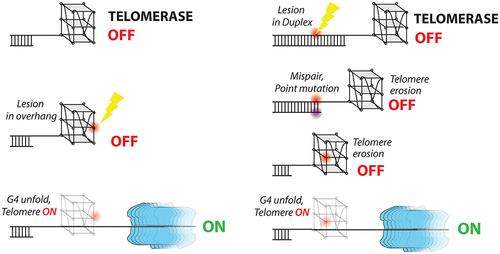
Telomeres are hot spots for mutagenic oxidative and methylation base damage due to their high guanine content. We used single-molecule fluorescence resonance energy transfer detection and biochemical assays to determine how different positions and types of guanine damage and mutations alter telomeric G-quadruplex structure and telomerase activity. We compared 15 modifications, including 8-oxoguanine (8oxoG), O-6-methylguanine (O6mG), and all three possible point mutations (G to A, T, and C) at the 3′ three terminal guanine positions of a telomeric G-quadruplex, which is the critical access point for telomerase. We found that G-quadruplex structural instability was induced in the order C < T < A ≤ 8oxoG < O6mG, with the perturbation caused by O6mG far exceeding the perturbation caused by other base alterations. For all base modifications, the central G position was the most destabilizing among the three terminal guanines. While the structural disruption by 8oxoG and O6mG led to concomitant increases in telomerase binding and extension activity, the structural perturbation by point mutations (A, T, and C) did not, due to disrupted annealing between the telomeric overhang and the telomerase RNA template. Repositioning the same mutations away from the terminal guanines caused both G-quadruplex structural instability and elevated telomerase activity. Our findings demonstrate how a single-base modification drives structural alterations and telomere lengthening in a position-dependent manner. Furthermore, our results suggest a long-term and inheritable effect of telomeric DNA damage that can lead to telomere lengthening, which potentially contributes to oncogenesis.
中文翻译:

鸟嘌呤碱基损伤和突变对端粒 G-四链体和端粒酶延伸的位置依赖性影响。
由于其高鸟嘌呤含量,端粒是诱变氧化和甲基化碱基损伤的热点。我们使用单分子荧光共振能量转移检测和生化分析来确定不同位置和类型的鸟嘌呤损伤和突变如何改变端粒 G-四链体结构和端粒酶活性。我们比较了 15 种修饰,包括 8-氧鸟嘌呤 (8oxoG)、O-6-甲基鸟嘌呤 (O6mG) 以及端粒 G-四链体的 3' 三个末端鸟嘌呤位置处的所有三种可能的点突变(G 到 A、T 和 C),这是端粒酶的关键接入点。我们发现 G-四链体结构不稳定性的诱导顺序为 C < T < A ≤ 8oxoG < O6mG,O6mG 引起的扰动远远超过其他碱基改变引起的扰动。对于所有碱基修饰,中央 G 位置是三个末端鸟嘌呤中最不稳定的。虽然 8oxoG 和 O6mG 的结构破坏导致端粒酶结合和延伸活性的伴随增加,但由于端粒悬垂和端粒酶 RNA 模板之间的退火中断,点突变(A、T 和 C)引起的结构扰动没有。将相同的突变从末端鸟嘌呤重新定位会导致 G-四链体结构不稳定和端粒酶活性升高。我们的研究结果证明了单碱基修饰如何以位置依赖的方式驱动结构改变和端粒延长。此外,我们的结果表明端粒 DNA 损伤的长期和可遗传影响可导致端粒延长,这可能有助于肿瘤发生。
更新日期:2020-07-21
中文翻译:

鸟嘌呤碱基损伤和突变对端粒 G-四链体和端粒酶延伸的位置依赖性影响。
由于其高鸟嘌呤含量,端粒是诱变氧化和甲基化碱基损伤的热点。我们使用单分子荧光共振能量转移检测和生化分析来确定不同位置和类型的鸟嘌呤损伤和突变如何改变端粒 G-四链体结构和端粒酶活性。我们比较了 15 种修饰,包括 8-氧鸟嘌呤 (8oxoG)、O-6-甲基鸟嘌呤 (O6mG) 以及端粒 G-四链体的 3' 三个末端鸟嘌呤位置处的所有三种可能的点突变(G 到 A、T 和 C),这是端粒酶的关键接入点。我们发现 G-四链体结构不稳定性的诱导顺序为 C < T < A ≤ 8oxoG < O6mG,O6mG 引起的扰动远远超过其他碱基改变引起的扰动。对于所有碱基修饰,中央 G 位置是三个末端鸟嘌呤中最不稳定的。虽然 8oxoG 和 O6mG 的结构破坏导致端粒酶结合和延伸活性的伴随增加,但由于端粒悬垂和端粒酶 RNA 模板之间的退火中断,点突变(A、T 和 C)引起的结构扰动没有。将相同的突变从末端鸟嘌呤重新定位会导致 G-四链体结构不稳定和端粒酶活性升高。我们的研究结果证明了单碱基修饰如何以位置依赖的方式驱动结构改变和端粒延长。此外,我们的结果表明端粒 DNA 损伤的长期和可遗传影响可导致端粒延长,这可能有助于肿瘤发生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号