当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Size-Resolved Chemical Composition of Sub-20 nm Particles from Methanesulfonic Acid Reactions with Methylamine and Ammonia
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-06-24 , DOI: 10.1021/acsearthspacechem.0c00120 Véronique Perraud 1 , Xiaoxiao Li 1, 2 , Jingkun Jiang 2 , Barbara J. Finlayson-Pitts 1 , James N. Smith 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-06-24 , DOI: 10.1021/acsearthspacechem.0c00120 Véronique Perraud 1 , Xiaoxiao Li 1, 2 , Jingkun Jiang 2 , Barbara J. Finlayson-Pitts 1 , James N. Smith 1
Affiliation
|
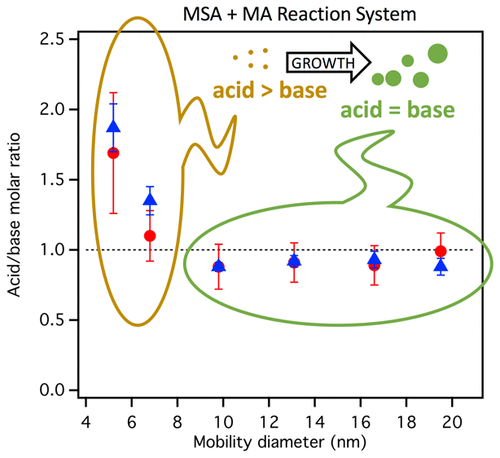
Particle formation in the atmosphere from gas-phase precursors has been observed around the world; however, our fundamental understanding of the key species responsible and mechanisms involved remains uncertain. Recent laboratory studies demonstrated that acid–base reactions involving methanesulfonic acid (CH3SO3H, MSA) and small alkylamines may contribute significantly to new particle formation. To date, most of the investigations have been focused on particle number concentration and size distribution measurements in combination with quantum chemistry predictions of the most stable clusters. Here, we present the first measurements of the size-resolved chemical composition of sub-20 nm particles generated in a custom flow reactor from the reaction of MSA with methylamine (MA) in the presence or absence of NH3 using thermal desorption chemical ionization mass spectrometry (TDCIMS). A novel design of the TDCIMS inlet was evaluated, and the measurement of the chemical composition of particles was extended down to 5 nm in diameter, the smallest size yet reported for this method. MSA–MA particles with diameters smaller than 9 nm were found to be more acidic, with an acid/base molar ratio of 1.8 ± 0.4 (1σ) for 5 nm particles compared to the larger particles which were neutral. A similar acid/base molar ratio trend was observed when NH3 was added to the MSA–MA combination. In the MSA–MA–NH3 system, the MA/NH3 molar ratio was higher than 1 (up to 2.6) for all particle sizes despite the much larger concentration of NH3 in the gas phase (the gas-phase MA/NH3 ratio was ∼0.23), indicating that MA is a key component in particle formation from this system. The potential reasons for this based on previous calculations of small clusters in this system are discussed.
中文翻译:

甲磺酸与氨和氨反应的亚20纳米颗粒的尺寸分辨化学组成
在世界范围内已经观察到由气相前驱物在大气中形成的颗粒。但是,我们对负责的关键物种和涉及的机制的基本了解仍不确定。最近的实验室研究表明,涉及甲磺酸(CH 3 SO 3H,MSA)和小的烷基胺可能会大大促进新颗粒的形成。迄今为止,大多数研究都集中在结合最稳定簇的量子化学预测的颗粒数浓度和尺寸分布测量上。在这里,我们介绍了在定制流动反应器中,在有或没有NH 3的情况下,MSA与甲胺(MA)反应生成的小于20 nm颗粒的尺寸分辨化学成分的首次测量使用热脱附化学电离质谱(TDCIMS)。评估了TDCIMS进样口的新颖设计,并将颗粒化学成分的测量范围扩展至直径5 nm,这是该方法迄今所报道的最小尺寸。发现直径小于9 nm的MSA–MA颗粒更酸性,与中性的较大颗粒相比,5 nm颗粒的酸/碱摩尔比为1.8±0.4(1σ)。将NH 3加入MSA-MA组合物中时,观察到类似的酸/碱摩尔比趋势。在MSA–MA–NH 3系统中,尽管NH 3浓度高得多,但所有粒径的MA / NH 3摩尔比均高于1(最高2.6)在气相中(气相MA / NH 3比为〜0.23),表明MA是该系统中颗粒形成的关键成分。讨论了基于此系统中小集群的先前计算的潜在原因。
更新日期:2020-07-16
中文翻译:

甲磺酸与氨和氨反应的亚20纳米颗粒的尺寸分辨化学组成
在世界范围内已经观察到由气相前驱物在大气中形成的颗粒。但是,我们对负责的关键物种和涉及的机制的基本了解仍不确定。最近的实验室研究表明,涉及甲磺酸(CH 3 SO 3H,MSA)和小的烷基胺可能会大大促进新颗粒的形成。迄今为止,大多数研究都集中在结合最稳定簇的量子化学预测的颗粒数浓度和尺寸分布测量上。在这里,我们介绍了在定制流动反应器中,在有或没有NH 3的情况下,MSA与甲胺(MA)反应生成的小于20 nm颗粒的尺寸分辨化学成分的首次测量使用热脱附化学电离质谱(TDCIMS)。评估了TDCIMS进样口的新颖设计,并将颗粒化学成分的测量范围扩展至直径5 nm,这是该方法迄今所报道的最小尺寸。发现直径小于9 nm的MSA–MA颗粒更酸性,与中性的较大颗粒相比,5 nm颗粒的酸/碱摩尔比为1.8±0.4(1σ)。将NH 3加入MSA-MA组合物中时,观察到类似的酸/碱摩尔比趋势。在MSA–MA–NH 3系统中,尽管NH 3浓度高得多,但所有粒径的MA / NH 3摩尔比均高于1(最高2.6)在气相中(气相MA / NH 3比为〜0.23),表明MA是该系统中颗粒形成的关键成分。讨论了基于此系统中小集群的先前计算的潜在原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号