当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultralong-Circulating and Self-Targeting "Watson-Crick A = T"-Inspired Supramolecular Nanotheranostics for NIR-II Imaging-Guided Photochemotherapy.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-06-24 , DOI: 10.1021/acsami.0c09090 Kaihang Xue 1 , Haina Tian 1 , Fukai Zhu 1 , Fanfan Wang 1 , Zhongxiong Fan 1 , Qingliang Zhao 2 , Zhenqing Hou 1, 3 , Yang Li 4, 5
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-06-24 , DOI: 10.1021/acsami.0c09090 Kaihang Xue 1 , Haina Tian 1 , Fukai Zhu 1 , Fanfan Wang 1 , Zhongxiong Fan 1 , Qingliang Zhao 2 , Zhenqing Hou 1, 3 , Yang Li 4, 5
Affiliation
|
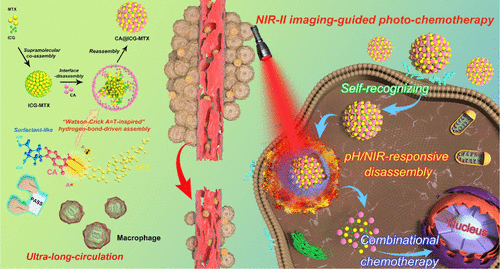
A carrier-free theranostic nanodrug directly coassembled using a NIR probe and a chemotherapeutic drug is a promising alternative for cancer theranostics. Nevertheless, this nanodrug still faces the limitations of short blood circulation and inefficient tumor accumulation/tumoral cellular uptake in vivo. Meanwhile, most exogenous targeting ligands and poly(ethylene glycol) have no therapeutic effect. Herein, we designed an ultralong-circulating and self-targeting nanodrug by an ordered supramolecular coassembly of indocyanine green (ICG), methotrexate (MTX, chemotherapeutic drug and cancer-cell-specific ligand), and clofarabine (CA). Notably, CA, as a surfactant-like chemotherapeutic drug, was introduced into the initial ICG-MTX coassembly by “Watson–Crick A = T-inspired” hydrogen-bond-driven sequential assembly with MTX. This carrier-free theranostic nanodrug with exceptionally high drug payload (100 wt %) not only showed superior serum stabilities but also displayed ultralong blood circulation (>7 days), enabling efficient accumulation at tumor sites. Moreover, our nanodrugs could be self-recognized by cancer cells and release the drugs on demand through lysosomal acidity and external laser stimulus. Under NIR-II imaging guidance, high-efficiency tumor ablation via synergistic photothermal-chemotherapy could be achieved in one treatment cycle while preventing the tumor recurrence. Our ultralong-circulating and self-recognizing carrier-free theranostic nanodrug based on the “drug-delivering-drug” strategy might have the potential for clinical theranostic application.
中文翻译:

用于NIR-II成像引导的光化学疗法的超长循环和自靶向“ Watson-Crick A = T”启发的超分子纳米化学。
使用NIR探针和化学治疗药物直接组装的无载体治疗药物纳米药物是癌症治疗药物的有希望的替代方法。然而,这种纳米药物仍然面临血液循环短和体内肿瘤积累/肿瘤细胞摄取效率低下的局限性。同时,大多数外源靶向配体和聚乙二醇没有治疗作用。在这里,我们通过吲哚菁绿(ICG),氨甲蝶呤(MTX,化疗药物和癌细胞特异性配体)和氯法拉滨(CA)的有序超分子共组装设计了超长循环和自靶向纳米药物。值得注意的是,CA作为一种表面活性剂类化学治疗药物,通过“沃森-克里克A = T启发”与MTX的氢键驱动顺序组装引入了最初的ICG-MTX组装。这种不含载体的治疗药物纳米药物具有极高的药物有效负载量(100 wt%),不仅显示出优异的血清稳定性,而且还显示出超长的血液循环(> 7天),从而能够在肿瘤部位有效积聚。此外,我们的纳米药物可以被癌细胞自我识别,并通过溶酶体酸度和外部激光刺激按需释放药物。在NIR-II成像指导下,可以通过协同光热化学疗法在一个治疗周期内实现高效肿瘤消融,同时防止肿瘤复发。我们基于“药物输送药物”策略的超长循环和自我识别的无载体治疗药物纳米药物可能具有临床治疗药物应用的潜力。
更新日期:2020-07-22
中文翻译:

用于NIR-II成像引导的光化学疗法的超长循环和自靶向“ Watson-Crick A = T”启发的超分子纳米化学。
使用NIR探针和化学治疗药物直接组装的无载体治疗药物纳米药物是癌症治疗药物的有希望的替代方法。然而,这种纳米药物仍然面临血液循环短和体内肿瘤积累/肿瘤细胞摄取效率低下的局限性。同时,大多数外源靶向配体和聚乙二醇没有治疗作用。在这里,我们通过吲哚菁绿(ICG),氨甲蝶呤(MTX,化疗药物和癌细胞特异性配体)和氯法拉滨(CA)的有序超分子共组装设计了超长循环和自靶向纳米药物。值得注意的是,CA作为一种表面活性剂类化学治疗药物,通过“沃森-克里克A = T启发”与MTX的氢键驱动顺序组装引入了最初的ICG-MTX组装。这种不含载体的治疗药物纳米药物具有极高的药物有效负载量(100 wt%),不仅显示出优异的血清稳定性,而且还显示出超长的血液循环(> 7天),从而能够在肿瘤部位有效积聚。此外,我们的纳米药物可以被癌细胞自我识别,并通过溶酶体酸度和外部激光刺激按需释放药物。在NIR-II成像指导下,可以通过协同光热化学疗法在一个治疗周期内实现高效肿瘤消融,同时防止肿瘤复发。我们基于“药物输送药物”策略的超长循环和自我识别的无载体治疗药物纳米药物可能具有临床治疗药物应用的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号