当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of new‐type 1,3,6‐triazocine via intramolecular reactions of iodocyclization and [3+2] azido cycloaddition
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-06-24 , DOI: 10.1002/jhet.4028 Ruslan I. Vaskevych 1, 2 , Alla I. Vaskevych 1, 3 , Eduard B. Rusanov 1 , Oksana Ya. Mel'nyk 4 , Mykhailo V. Vovk 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-06-24 , DOI: 10.1002/jhet.4028 Ruslan I. Vaskevych 1, 2 , Alla I. Vaskevych 1, 3 , Eduard B. Rusanov 1 , Oksana Ya. Mel'nyk 4 , Mykhailo V. Vovk 1
Affiliation
|
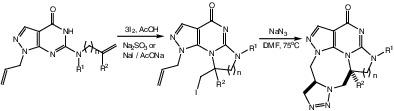
Selective iodocyclization of 6‐(alkenylamino)‐1‐allylpyrazolo[3,4‐d ]pyrimidines provided hydrogenated derivatives of 1‐allyl‐8(9)‐iodomethylimidazo(pyrimido)[1,2‐a ]pyrazolo[4,3‐e ]pyrimidines which were further reacted with NaN3 at 75°С to 80°С to give a series of new‐type 1,3,6‐triazocines annulated with the pyrazole, pyrimidine, imidazole (or pyrimidine), and 1,2,3‐triazole rings. The compounds synthesized were structurally characterized by analytical, spectral (IR, 1H and 13C NMR, HPLC‐mass), and X‐ray diffraction data.
中文翻译:

通过碘环化和[3 + 2]叠氮基环加成反应的分子内反应合成新型1,3,6-三唑嗪
6-(烯基氨基)-1-烯丙基吡唑并[ 3,4- d ]嘧啶的选择性碘环化提供了1-烯丙基-8(9)-碘甲基咪唑并(pyrimido)[1,2- a ]吡唑并[4,3- e ]嘧啶,在75°C至80°C下与NaN 3进一步反应,得到一系列新型1,3,6-三唑嗪,与吡唑,嘧啶,咪唑(或嘧啶)和1,2 ,3-三唑环 通过分析,光谱(IR,1 H和13 C NMR,HPLC质量)和X射线衍射数据对合成的化合物进行结构表征。
更新日期:2020-08-08
中文翻译:

通过碘环化和[3 + 2]叠氮基环加成反应的分子内反应合成新型1,3,6-三唑嗪
6-(烯基氨基)-1-烯丙基吡唑并[ 3,4- d ]嘧啶的选择性碘环化提供了1-烯丙基-8(9)-碘甲基咪唑并(pyrimido)[1,2- a ]吡唑并[4,3- e ]嘧啶,在75°C至80°C下与NaN 3进一步反应,得到一系列新型1,3,6-三唑嗪,与吡唑,嘧啶,咪唑(或嘧啶)和1,2 ,3-三唑环 通过分析,光谱(IR,1 H和13 C NMR,HPLC质量)和X射线衍射数据对合成的化合物进行结构表征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号